Abstract
Extracellular histones have been reported to aggravate different pathophysiological processes by increasing vascular permeability, coagulopathy, and inflammation. In the present study, we elucidate how extracellular histones (10–100 µg/mL) concentration dependently increase cytosolic reactive oxygen species (ROS) production using human umbilical vein endothelial cells (HUVECs). Furthermore, we identify cyclooxygenase (COX) and NADPH oxidase (NOX) activity as sources of ROS production in extracellular histone-treated HUVEC. This COX/NOX-mediated ROS production is also involved in enhanced NF-kB activity and cell adhesion molecules (VCAM1 and ICAM1) expression in histone-treated HUVEC. Finally, by using different toll-like receptor (TLR) antagonists, we demonstrate the role of TLR4 in CAMs overexpression triggered by extracellular histones in endothelial cells. In conclusion, our data suggest that through TLR4 signaling, extracellular histones increase endothelial cell activation, a mechanism involving increased COX- and NOX-mediated ROS. These findings increase our understanding on how extracellular histones enhance systemic inflammatory responses in diseases in which histone release occurs as part of the pathological processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histones are essential proteins regulating chromatin conformation and gene transcription, but when histones are released into extracellular space, they can mediate proinflammatory activity [3]. Extracellular histones act as an aggravating factor in multiple pathophysiological processes and disease progression [7]. Among these, they have been implicated in organ injury after trauma [1], autoimmune diseases [21], and ischemic heart disease [5]. In addition, when released into the bloodstream, histones mediate in the pathology of strokes [9], disseminated intravascular coagulation [23], sepsis [32], and septic shock [11].
As a communicative dynamic barrier between intravascular and extravascular spaces, endothelium is responsive to circulating compounds, including extracellular histones. Indeed, it has been reported that organ injury mediated by extracellular histones is caused primarily through endothelial damage [18] and induced endothelial barrier dysfunction [12]. Specifically, molecular studies in histone-exposed endothelial cells have pinpointed their roles in inducing Ca2+ overload [8], increasing cell adhesion molecules (CAMs) [35] and tissue factor expression [34], and disarranging vasoactive mediator release [25], thus altering vascular homeostasis. In addition, both apoptotic and autophagy pathway activation have been implicated in histone-mediated endothelial cell death [16].
Dying cells and neutrophil extracellular trap (NET) formation by activated neutrophils have been shown to be major endogenous sources of extracellular histones [7, 10]. These histones and nucleosomes released into the bloodstream are described as damage-associated molecular patterns (DAMPs) [14] whose response is driven by pattern recognition receptors (PPRs) [4]. Among PPRs, toll-like receptors (TLRs) have been reported as the main receptors for extracellular histones [7]. In this regard, endothelium expresses different TLRs [31], which modify its physiology upon exposure to extracellular histones, ultimately resulting in endothelial injury and dysfunction [7].
Extracellular histones have been implicated in increased production of reactive oxygen species (ROS) [17, 27]. Production of ROS has been identified as a key component in progression of many inflammatory diseases, acting both as signaling molecule and inflammatory mediator [20]. Although recent data have provided insight into several actions triggered by extracellular histones in endothelial cells, the mechanisms by which extracellular histones increase ROS production remain unclear.
Although previous studies have reported that extracellular histones increase intracellular ROS levels in human umbilical vein endothelial cells (HUVEC) [25], the sources of ROS involved in histone-triggered effects have been less studies. Here, we aimed to get deeper insight into the mechanism involved in the production of oxidative stress in HUVEC exposed to extracellular histones, interrogating for the main oxidative stress generating cellular factors. Furthermore, we investigated the role of increased ROS production in the activity of key inflammatory modulator NF-kB, and expression of different CAMs in human endothelial cells exposed to extracellular histones. Finally, we determined the role of different TLR types in the observed histone-triggered response.
Methods
Cell culture and experimental design
Pooled human umbilical vein endothelial cells (HUVECs) from 5 individual female donors were purchased from Lonza (Barcelona, Spain) and were grown in Medium 199 (Sigma-Aldrich, Madrid, Spain) supplemented with 20% fetal bovine serum (Gibco, Invitrogen, Barcelona, Spain), endothelial cell growth supplement from bovine neural tissue (ECGS, Sigma-Aldrich), and heparin sodium salt from porcine intestinal mucosa (Sigma-Aldrich). Cells were routinely grown in an incubator at 37 °C with 5% CO2. HUVECs from passages 3 to 5 were used in this study. When they reached confluence, the media was changed and cells underwent 4 h exposure to different calf thymus histone concentrations (Sigma-Aldrich, St. Louis, MO, USA): 10, 25, 50, or 100 µg/mL prepared in PBS. In some experiments, 30 µM apocynin (Sigma-Aldrich), 10 µM indomethacin (Sigma-Aldrich), 10 µM celecoxib (Sigma-Aldrich), 100 µmol/l tempol (Sigma-Aldrich), 20 µM Bay11-7082 ((E)-3-(4-methylphenylsulfonyl)-2-propenenitrile; Sigma-Aldrich), 20 µg/mL oxPAPC (Invivogen, Toulouse, France), 0.7 µM iODN (inhibitory oligodeoxynucleotide with phosphorothioate backbone, Enzo Life Science, Farmingdale, USA), and 3 µM CLI-095 (Invivogen) were added to HUVEC 1 h prior to histone treatment.
Reactive oxygen species production measurement
Intracellular reactive oxygen species (ROS) production was detected using fluorescence probes: dihydroethidium (DHE, Invitrogen) for intracellular ROS or MitoSOX (Invitrogen) for mitochondrial ROS. Histone-treated cells were loaded with 2.5 μM DHE or 5 μM MitoSOX for 30 min. Next, cells were rinsed with PBS and observed under an inverted fluorescence Nikon Eclipse Ti-S microscope. Fluorescence was measured from three different fields per well. Fluorescence signals were quantified using NIS-Elements 3.2 software (Nikon Izasa S.A, L’Hospitalet de Llobregat, Spain).
Cell transfection
HUVECs were cultured overnight before being transfected with Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific). Short interference (si)RNA negative control (Assay ID 117,432) and siRNA NOX1 inhibitor (Assay ID 4,390,843) were used at 20 nM in serum-free OptiMEM medium (Gibco) during 48 h. HUVEC transfected with siNOX1 showed ~ 40% reduction in NOX1 protein levels (Suppl. Figure 1B).
Gene expression analysis by RT-qPCR
Total RNA was isolated from cells using NucleoSpin® RNA/Protein (740,933.50, Macherey–Nagel, Düren, Germany) according to the manufacturer’s instructions. For reverse transcription (RT) reactions, 200 ng of purified RNA was reverse transcribed using random hexamers with the high-capacity cDNA reverse transcription kit (P/N 4,322,171, Applied Biosystems, Foster City, USA) according to the manufacturer’s instructions.
Gene expression was determined by quantitative real-time PCR analysis using an ABI Prism 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Gene-specific primer pairs and probes were purchased from Applied Biosystems (Assays-on-Demand) for SOD1 (Hs00533490_m1), SOD2 (Hs00167309_m1), VCAM1 (Hs01003372_m1), ICAM1 (Hs00164932_m1), and GAPDH (Hs99999905_m1), and were used together with TaqMan Universal PCR Master Mix (P/N 4,304,437) and reverse-transcribed sample RNA in 20-μl reaction volumes. iTaq TM Universal SYBR Green supermix (Bio-Rad Laboratories Inc., Madrid, Spain) was also used in order to determine mRNA expression. Primers used in SYBR green-based qRT-PCR were all purchased from Sigma-Aldrich. Primers sequences are described in Table 1. PCR conditions were determined according with manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase expression levels were measured in all samples to normalize differences in RNA input, RNA quality, and reverse transcription efficiency. Each sample was analyzed in triplicate, and expression was calculated according to the 2−ΔΔCt method.
Protein expression analyzed by Western blot
Proteins extracts (50 μg) were denatured with sample buffer (Tris 40 mM, EDTA, bromophenol blue 0.01%, sucrose 40%, SDS 4%, β-mercaptoethanol 10%) and heated to 95 °C for 5 min. Afterwards, samples were electrophoresed in 12% SDS-PAGE and transferred onto nitrocellulose membrane (Whatman GmbH, Dassel, Germany).
After transference, the membrane was blocked with 5% milk or 5% BSA (in the case of phosphorylated proteins) in Tris-buffered saline and Tween 20 (TBST) for 1 h. Afterwards, the membranes were incubated with the specific primary antibodies: p65 subunit of NF-κB, p-p65(Ser276), SOD1, and SOD2 (from Cell Signaling, Beverly, MA, USA); NLRP3 (from Novus, NBP2-12,446); COX1, COX-2, NOX1, NOX4, and β-actin as loading control (from Santa Cruz BioTech, Dallas, TX, USA).
Afterwards, the blots were washed again with TBST and incubated for a further 1 h with a secondary mouse, rabbit, or goat antibody with horseradish peroxidase-linked conjugate. The membrane was incubated at room temperature with constant agitation. Finally, the membrane was washed 3 × 5 min with TBST. Luminol was added onto the membrane (ECL Western Blotting Detection Reagents, GE Healthcare, Hatfield, and Hertfordshire, UK), and membrane chemiluminescence was revealed by LAS-4000 image reader (GE Healthcare).
Statistical analysis
Values are expressed as mean ± standard error of mean (SEM). Student’s t-test was applied for between-group comparisons. One-way analysis of variance was used to determine the difference between groups. When an interaction effect was found, multiple comparisons were performed using the Student–Newman–Keuls method “post hoc” test. Statistical significance was set at *P < 0.05, **P < 0.01, and ***P < 0.001, as indicated in each case. GraphPad Prism v6.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis and graphic representations.
Results
Extracellular histones induce ROS production in a concentration-dependent manner and increase antioxidant enzyme expression in HUVEC
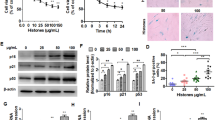
The first aim of the present work was to investigate the effect of extracellular histones on ROS production in endothelial cells. Exposure of HUVEC to increasing concentrations of extracellular histones (10, 25, 50, and 100 μg/mL) for 4 h resulted in a concentration-dependent increase of ROS production, showing statistically significance with concentrations above 50 μg/mL. Specifically, ROS production increased up to 80 ± 10% when cells were exposed to 50 μg/mL (P < 0.001), and up to 103 ± 19% when exposed to 100 μg/mL (P < 0.001) (Fig. 1A). Conversely, no changes in mitochondrial ROS production were observed in endothelial cells exposed to 50 μg/mL of extracellular histones compared to non-treated cells (Fig. 1B).
Extracellular histone-treated HUVEC increases ROS production and antioxidant response through increased SOD1 expression. A HUVECs were exposed to different concentrations of histones for 4 h and intracellular ROS levels were determined by DHE oxidation (n = 7) as described in Methods. B HUVECs were exposed to 0 µg/mL of histones (0H) and 50 µg/mL of histones (50H) for 4 h and mitochondrial ROS levels were determined by MitoSOX probe (n = 6) as described in Methods. C HUVECs were exposed to 50 µg/mL of histones for 4 h (n = 3). Relative SOD1 and SOD2 expression were determined by qRT-PCR. D Protein extracts (20 µg protein) from cultured HUVEC incubated at 50 µg/mL of histones for 4 h were loaded on SDS-PAGE gels and analyzed by Western blotting using anti-SOD1 and anti-SOD2. β-actin was used as loading control. One representative experiment of three performed is shown. Relative levels assessed by densitometry are presented. Data are expressed as mean ± SEM of n = 6–7. *P < 0.05 and ***P < 0.001 versus 0 µg/mL of histones
Furthermore, HUVEC exposed to extracellular histones (50 μg/mL) showed altered expression of cytosolic superoxide dismutase (SOD1), whereas no significant differences were observed for the mitochondrial variant SOD2. Results showed significantly increased SOD1 expression levels in HUVEC (21 ± 8%, P < 0.05; Fig. 1C) at 50 μg/mL of extracellular histones. These data were supported by protein levels: relative levels assessed by densitometry revealed a significant increase in the cytosolic SOD (82 ± 35%, P < 0.05), while conversely, mitochondrial SOD protein levels were found unaltered (Fig. 1D) in agreement to the mRNA levels measured by RT-qPCR.
COX and NOX are involved in ROS production induced by extracellular histones in HUVEC
We next sought to determine which specific sources of ROS production were induced in endothelial cells exposed to extracellular histones. NADPH-oxidase (NOX) [6] and cyclooxygenase (COX) [26] have been suggested as important sources of ROS production under inflammatory conditions. To evaluate the source of ROS production of histone-treated HUVEC, we incubated HUVEC with apocynin (antioxidant) and indomethacin (COX inhibitor) before extracellular histone (50 μg/mL) treatment. Results showed a significant decrease in ROS production when endothelial cells were treated with both apocynin and indomethacin. (Fig. 2A). In addition, to determine the role of extracellular histones in modulation of different isoforms of COX and NOX, HUVECs were treated with 50 μg/mL extracellular histones, and COX-1, COX-2, NOX1, and NOX4 mRNA expression and protein levels were determined. Histone-exposed HUVEC showed increased mRNA expression of COX-2 and NOX1, while COX-1 and NOX4 expression remained unaltered (Fig. 2B). Moreover, histones induced an increase in COX-2 protein production (38 ± 6%, P < 0.001) while COX-1 levels were unaltered. Conversely, no significant changes on NOX1 and NOX4 were observed (Fig. 2C). Altogether, these results suggest a role for COX and NOX enzymes in histone-dependent ROS production in HUVEC.
ROS induced by extracellular histones is mediated by COX and NOX activity. A HUVECs were preincubated with apocynin (Apo) and indomethacin (Indo) for 1 h and treated with 0 µg/mL of histones (0H) and 50 µg/mL of histones for 4 h (50H, n = 10). Intracellular ROS levels were determined by DHE oxidation as described in Methods. B HUVEC exposed to 50 µg/mL of histones for 4 h (n = 7). Relative COX-1, COX-2, NOX1, and NOX4 expression were determined by qRT-PCR. C Protein extracts (20 µg protein) from cultured HUVEC incubated at 50 µg/mL of histones for 4 h (n = 4) were loaded on SDS-PAGE gels and analyzed by Western blotting using anti-COX-1, anti-COX-2, anti-NOX1, and anti-NOX4. β-actin was used as loading control. One representative experiment of three performed is shown. Relative levels assessed by densitometry are presented. Data are expressed as mean ± SEM. *P < 0.05 and ***P < 0.001 versus 0 µg/mL of histones
ROS productions mediated by COX and NOX are involved in NF-kB/CAM pathway expression in histone-treated HUVEC
Given that increased ROS levels are associated with increased activity of pro-inflammatory mediators and with the expression of adhesion molecules, we next performed Western blot analysis to evaluate the levels and activity of NF-κB, a pivotal molecule in endothelial inflammation control, and the expression and activity of VCAM1 and ICAM1, both involved in leukocyte adhesion to endothelium monolayer.
Figure 3A shows a concentration-dependent increase of the p65 subunit of NF-κB and its phosphorylation in HUVEC treated with extracellular histones. Blot quantification revealed a significant increase of p65 phosphorylation when endothelial cells were exposed to 50 (P < 0.05) and 100 μg/ml (P < 0.01) of extracellular histones (Fig. 3A). Importantly, this effect was abrogated when cells were previously exposed to BAY11-7082, an inhibitor of p65 activation (Fig. 3B). Since Bay 11–7082 also acts as a selective inhibitor for nod-like receptor family pyrin domain containing 3 (NLRP3), we also determined the NLRP3 protein levels. However, no significant changes were observed in HUVEC exposed to increasing concentrations of extracellular histones (Fig. 3B). Also, no changes were observed in the expression of the pro-inflammatory cytokines IL-1β, IL-18, and IL-1α associated with NLRP3 in histone-treated cells (Suppl. Figure 1A).
COX/NOX activity, via ROS production, is involved in NFkB-dependent VCAM1 and ICAM1 expression increase in extracellular histone-treated HUVEC. A Protein extracts (20 µg protein) from HUVEC exposed to different concentrations of histones for 4 h (n = 4) were loaded on SDS-PAGE gels and analyzed by Western blotting using anti-p-p65, anti-p65, and anti-NLRP3. β-actin was used as loading control. One representative experiment of three performed is shown. Relative levels assessed by densitometry are presented. B HUVECs were incubated with Bay11-7082 (Bay) and exposed to 50 µg/mL of histones for 4 h (n = 4). Relative VCAM1 and ICAM1 expression were determined by qRT-PCR. C HUVECs were incubated with tempol, apocynin, and celecoxib, and exposed to 50 µg/mL of histones for 4 h (n = 3). Relative VCAM1 and ICAM1 expression were determined by qRT-PCR. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 versus 0 µg/mL of histones. #P < 0.05, ##P < 0.01 versus 50 µg/mL of histones
To investigate the effect of extracellular histones in VCAM1 and ICAM1 expression, we determined mRNA expression in HUVEC by qRT-PCR. Histone-treated HUVEC showed significant increased expression (Fig. 3B) of both VCAM1 (150 ± 20%, P < 0.01) and ICAM1 (177 ± 53%, P < 0.001) at 50 μg/mL of histones. As occurred for p65 activation, BAY11-7082 caused a significant reduction in histone-mediated VCAM1 (P < 0.05) and ICAM1 (P < 0.05) mRNA levels (Fig. 3C), indicating that NF-κB is involved in adhesion molecule expression induced by extracellular histones in HUVEC.
In order to determine whether extracellular histone-induced ROS-generating enzymes are related to the observed pro-inflammatory pathway induction, we studied VCAM1 and ICAM1 mRNA expression in histone-treated HUVEC previously incubated with the superoxide dismutase mimetic tempol, apocynin, and the COX-2-specific inhibitor celecoxib. All treatments abrogated the VCAM1 and ICAM1 mRNA induction observed in endothelial cells exposed to extracellular histones (Fig. 3D). Moreover, specific siRNA knock-down of NOX-1 significantly reduced ROS production and VCAM1 expression in histone-treated cells (Suppl. Figure 1B). These results thus indicate the involvement of ROS, NOX-1 and COX-2, in the enhanced CAM expression observed in endothelial cells after extracellular histone exposure.
TLR4 is involved in ROS-dependent CAM expression in extracellular histone-treated HUVEC
As described in the introduction, extracellular histones have been reported to bind to the cell surface through TLR receptors, although the specific TLR responsible for this process is still under debate [14, 33]. We found that preincubation of HUVEC with TLR inhibitors after extracellular histone treatment modulated ROS production (Fig. 4A). The results demonstrated that histone-induced ROS production decreased significantly upon preincubation with OxPAPC (a TLR2 and TLR4 inhibitor), but not when HUVECs were pre-treated with iODN (an TLR7 and TLR9 inhibitor). CLI-095, a selective TLR4 inhibitor, was used to determine the specificity of extracellular histone binding to this receptor in HUVEC. CLI-095 decreased histone-mediated ROS production in HUVEC (P < 0.01, CLI-095 + 50 μg/mL of histones relative to 50 μg/mL of histones) (Fig. 4A). In addition, expression of MYD88, an adapter protein that mediates signal transduction from TLRs to NF-κB, showed a trend for increased expression in HUVEC exposed to extracellular histones (50 μg/ml) compared to non-treated cells (Suppl. Figure 1C). Altogether, these results indicate that HUVECs exposed to extracellular histones exhibit an increase in ROS production via TLR4.
TLR4 is involved in ROS-dependent increase in VCAM1 and ICAM1 expression in histone-treated endothelial cells. HUVECs were preincubated with OxPAPC, iODN, and CLI-095 for 1 h and treated with 50 µg/mL of histones for 4 h (50H, n = 8). Intracellular ROS productions were determined by DHE oxidation as described in Methods. B HUVECs were incubated with CLI-095 and exposed to 50 µg/mL of histones for 4 h (n = 8). Relative VCAM1 and ICAM1 expression were determined by qRT-PCR. Data are expressed as mean ± SEM. **P < 0.01 and ***P < 0.001 versus 0 µg/mL of histones. #P < 0.05, ##P < 0.01 versus 50 µg/mL of histones. C Working model
We further analyzed the role of TLR4 in CAM expression in histone-treated HUVEC. Extracellular histone-treated endothelial cells were preincubated with CLI-095 and VCAM1 and ICAM1 mRNA expression (Fig. 4B) were determined. Results showed reduced VCAM1 and ICAM1 mRNA expression in histone-incubated cells pre-treated with TLR4 antagonist, thus reverting the induction produced by 50 µg/mL of histones.
Taken together, our experiments indicate that extracellular histones increase cytosolic oxidative status in HUVEC, increasing ROS production and altering antioxidant enzymes. ROSs are produced by COX and NOX enzymes after extracellular histone exposure via a TLR4-dependent mechanism, which in turn leads to heightened NF-kB activation and VCAM1 and ICAM1 expression (Fig. 4C).
Discussion
In this study, we demonstrated that endothelial cells exposed to extracellular histones enhance ROS production through a TLR4-COX/NOX pathway, which in turn increases cell adhesion molecules (VCAM1 and ICAM1) expression via NFkB activity. First, histone-treated HUVEC showed a concentration-dependent rise in cytosolic ROS production and a concomitant increase in the antioxidant cytosolic superoxide dismutase enzyme SOD1. Second, extracellular histone-induced superoxide anion production is mediated by COX and NOX activity. Third, COX/NOX-mediated increase in ROS induced VCAM1 and ICAM1 expression through an NFkB-dependent mechanism. Finally, we identified TLR4 as the main receptor involved in the above described pathway.
Extracellular histones contribute to the pathobiology of systemic inflammatory diseases in which endothelium activation seems to play a crucial, including both infections, such as sepsis [32], and sterile inflammation, such as stroke [9], disseminated intravascular coagulation [23], or ischemia–reperfusion injury [14]. Endothelial cells exposed to extracellular histones release proinflammatory cytokines [12], induce tissue factor expression [34], and show increased adhesion molecules in the cell membrane [35]. Indeed, in vivo experiments have demonstrated that administration of extracellular histone causes neutrophil migration, endothelial dysfunction, and thrombosis [32].
Our results demonstrate that extracellular histones increase ROS production in a concentration-dependent manner in endothelial cells. Production of ROS is vital in the pathogenesis of vascular injury and contributes to different vascular responses in inflammation, such as vasomotor dysfunction, impaired vascular permeability, enhanced thrombus formation, and leukocyte recruitment [19]. Increased ROS levels have also been observed in histone-treated cardiomyocytes [17] and Kuppfer cells [15]. Moreover, pretreatment of dendritic cells with antioxidants prevented H4-induced cytokine secretion [2]. Furthermore, histone-exposed endothelial cells showed increased expression of the cytosolic SOD1, which can be explained as an adaptive compensatory antioxidant mechanism in response to oxidative stress to maintain the redox-state balance [13].
Our results showed that histone-mediated ROS production depends on NOX and COX activity. In this regard, NOX-dependent overproduction of ROS observed in cardiac myocytes exposed to plasma from patients with sepsis [30] could be due to elevated circulating levels of pro-inflammatory mediators, including extracellular histones [11, 32]. Extracellular histones treatment of HUVEC did not change NOXs protein levels. In this regard, direct interaction of TLR4 with Nox4 has been reported as the mechanism involved in LPS-mediated ROS generation [24]. Furthermore, it has been reported that ROS production by NOX can subsequently trigger other ROS-generating sources [6] such as COX. Our findings indicate that extracellular histone treatment enhanced COX-2 expression while COX1 remained unaltered. In this regard, increased COX-2 expression has previously been observed in dermal microvascular endothelial cells exposed to P. falciparum histones [12]. As demonstrated by inhibiting COX-2 activity, ROS production should be mediated by COX-2 expression enhancement observed in histone-treated endothelial cells. In agreement with these results, multiple studies have focused on the contribution of COX-2-dependent oxidative stress in endothelial inflammation [29], suggesting its role as an inflammatory signal mediator.
The endothelium responds to inflammatory mediators by expressing adhesion molecules on the cell surface, increasing rolling, adherence, and transmigration of leukocytes into the underlying tissue. Here, we demonstrate elevated VCAM1 and ICAM1 expression in endothelial cells exposed to extracellular histones, which are known to contribute to inflammatory cell recruitment. These results agree with the previous findings of Shrestha et al. [27] and are in agreement with the results that histone-neutralizing antibodies significantly reduced neutrophil recruitment in an in vivo mice model of sterile inflammation [2]. Additionally, we show that the VCAM1 increased expression in histone-treated endothelial cells is dependent on NF-kB, a key inflammatory modulator whose activity can be regulated by the cellular redox status [22], in a concentration-dependent manner. Similar results have been shown using primary human coronary artery endothelial cells exposed to extracellular histones [34].
Our experiments using different TLR antagonists indicate that intervention on TLR4 can restore endothelial levels of ROS production enhanced by extracellular histone exposure, and hence levels of adhesion molecules in HUVEC. Several studies propose that extracellular histone action is triggered via TLRs [14, 33, 34], and our results reinforce this idea and further demonstrate that endothelial adhesion factors are stimulated via TLR4 in HUVEC. Extracellular histone release has been implicated in tissue factor expression in vascular endothelial cells via TLR2/4-dependent mechanisms [34]. In the liver, however, histone-induced tissue injury has been linked to activation of both TLR2/4 [33] and TLR9 [14]. Indeed, cell lineage could also be involved in TLR-mediated histone action, since it has been observed that extracellular histone-activated TLR9 leads to ROS production in Kupffer cells [15]. Furthermore, using KO mice, Xu et al. found that both TLR2 and TLR4 were implicated in histone-mediated cell death, but only TLR4 was responsible for histone-dependent increase of cytokines levels [33]. These results suggest that histone binding to specific TLRs could activate different molecular pathways that will result in a determinant response. Opposite to our results, histone-induced expression of adhesion molecules was inhibited by neutralizing antibodies anti-TLR9, but not by anti-TLR2 or anti-TLR4, suggesting that TLR9 is involved in the histone-induced induction of adhesion molecules in EA.hy926 endothelial cells [35]. Discrepancies could be due to the previously reported differences between the cell line EA.hy926 and HUVECs [28] and reinforce the idea that cell specificity may be related to TLR-mediated histone action.
In conclusion, our findings demonstrate that NOX and COX have a central role in enhanced ROS production exhibited in human endothelial cells exposed to extracellular histones. Furthermore, over-production of ROS in histone-treated HUVEC increases CAM expression in an NF-kB-dependent pathway, an effect which is triggered specifically through TLR4.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W et al (2013) Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 187:160–169
Allam R, Darisipudi MN, Tschopp J, Anders HJ (2013) Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol 43:3336–3342
Allam R, Kumar SV, Darisipudi MN, Anders HJ (2014) Extracellular histones in tissue injury and inflammation. J Mol Med (Berl) 92:465–472
Allen KS, Sawheny E, Kinasewitz GT (2015) Anticoagulant modulation of inflammation in severe sepsis. World J Crit Care Med 4:105–115
Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L et al (2013) Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 33:2032–2040
Brandes RP, Kreuzer J (2005) Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65:16–27
Chen R, Kang R, Fan XG, Tang D (2014) Release and activity of histone in diseases. Cell Death Dis 5:e1370
Collier DM, Villalba N, Sackheim A, Bonev AD, Miller ZD, Moore JS, Shui B, Lee JC, Lee FK, Reining S et al (2019) Extracellular histones induce calcium signals in the endothelium of resistance-sized mesenteric arteries and cause loss of endothelium-dependent dilation. Am J Physiol Heart Circ Physiol 316:H1309–H1322
De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD (2012) Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 32:1884–1891
García-Giménez JL, Romá-Mateo C, Seco-Cervera M, Ibañez-Cabellos JS & Pallardó FV (2016) Chapter 25 - circulating histones and nucleosomes as biomarkers in sepsis and septic shock In: José Luis García-Giménez (Ed), Epigenetic Biomarkers and Diagnostics, Academic Press, Boston:pp. 497–517
García-Giménez JL, Romá-Mateo C, Carbonell N, Palacios L, Peiró-Chova L, García-López E, García-Simón M, Lahuerta R, Gimenez-Garzó C, Berenguer-Pascual E et al (2017) A new mass spectrometry-based method for the quantification of histones in plasma from septic shock patients. Sci Rep 7:10643
Gillrie MR, Lee K, Gowda DC, Davis SP, Monestier M, Cui L, Hien TT, Day NP, Ho M (2012) Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am J Pathol 180:1028–1039
Haorah J, Floreani NA, Knipe B, Persidsky Y (2011) Stabilization of superoxide dismutase by acetyl-l-carnitine in human brain endothelium during alcohol exposure: novel protective approach. Free Radical Biol Med 51:1601–1609
Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT et al (2011) Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology (Baltimore, MD) 54:999–1008
Huang H, Chen H-W, Evankovich J, Yan W, Rosborough BR, Nace GW, Ding Q, Loughran P, Beer-Stolz D, Billiar TR et al (2013) Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J Immunol 191:2665–2679
Ibañez-Cabellos JS, Aguado C, Pérez-Cremades D, García-Giménez JL, Bueno-Betí C, García-López EM, Romá-Mateo C, Novella S, Hermenegildo C, Pallardó FV (2018) Extracellular histones activate autophagy and apoptosis via mTOR signaling in human endothelial cells. Biochimica et Biophysica Acta - Molecular Basis of Disease 1864:3234–3246
Kalbitz M, Grailer JJ, Fattahi F, Jajou L, Herron TJ, Campbell KF, Zetoune FS, Bosmann M, Sarma JV, Huber-Lang M et al (2015) Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J 29:2185–2193
Kawai C, Kotani H, Miyao M, Ishida T, Jemail L, Abiru H, Tamaki K (2016) Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol 186:829–843
Kvietys PR, Granger DN (2012) Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radical Biol Med 52:556–592
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167
Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, Wright J, Thornhill TS, Monestier M, Ploegh H et al (2009) A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci USA 106:15867–15872
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res 21:103–115
Nakahara M, Ito T, Kawahara K, Yamamoto M, Nagasato T, Shrestha B, Yamada S, Miyauchi T, Higuchi K, Takenaka T et al (2013) Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS ONE 8:e75961
Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS (2004) Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol (Baltimore, Md: 1950) 173:3589–3593
Perez-Cremades D, Bueno-Beti C, Garcia-Gimenez JL, Ibanez-Cabellos JS, Hermenegildo C, Pallardo FV, Novella S (2017) Extracellular histones disarrange vasoactive mediators release through a COX-NOS interaction in human endothelial cells. J Cell Mol Med 21:1584–1592
Shi Y, Vanhoutte PM (2008) Oxidative stress and COX cause hyper-responsiveness in vascular smooth muscle of the femoral artery from diabetic rats. Br J Pharmacol 154:639–651
Shrestha C, Ito T, Kawahara K, Shrestha B, Yamakuchi M, Hashiguchi T, Maruyama I (2013) Saturated fatty acid palmitate induces extracellular release of histone H3: a possible mechanistic basis for high-fat diet-induced inflammation and thrombosis. Biochem Biophys Res Commun 437:573–578
Thornhill MH, Li J, Haskard DO (1993) Leucocyte endothelial cell adhesion: a study comparing human umbilical vein endothelial cells and the endothelial cell line EA-hy-926. Scand J Immunol 38:279–286
Vidal-Gomez X, Novella S, Perez-Monzo I, Garabito M, Dantas AP, Segarra G, Hermenegildo C, Medina P (2016) Decreased bioavailability of nitric oxide in aorta from ovariectomized senescent mice. Role of cyclooxygenase. Exp Gerontol 76:1–8
Wu J, Xu H, Yang M, Martin CM, Kvietys PR, Rui T (2009) NADPH oxidase contributes to conversion of cardiac myocytes to a proinflammatory phenotype in sepsis. Free Radical Biol Med 46:1338–1345
Xiao L, Liu Y, Wang N (2013) New paradigms in inflammatory signaling in vascular endothelial cells. Am J Physiol-Heart Circ Physiol 306:H317–H325
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT (2009) Extracellular histones are major mediators of death in sepsis. Nat Med 15:1318–1321
Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT (2011) Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol (Baltimore, Md: 1950) 187:2626–2631
Yang X, Li L, Liu J, Lv B, Chen F (2016) Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb Res 137:211–218
Yoo HJ, Lee J-S, Kim J-E, Gu J, Koh Y, Kim I, Kim HK (2016) Extracellular histone released from leukemic cells increases their adhesion to endothelium and protects them from spontaneous and chemotherapy-induced leukemic cell death. PLoS ONE 11:e0163982
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by grants from the Spanish Ministerio de Ciencia, e Innovación, Instituto de Salud Carlos III—FEDER-ERDF (PI19/01714; PI19/00994), Generalitat Valenciana (CIAICO/2021/211), and the COST Action (CA17129: CardioRNA), CaixaImpulse (LCF/TR/CI18/50030003) grant by Fundació la Caixa, and Research Projects grants by Fundación Mutua Madrileña (AP174352020). JLG-G and FVP thank the Spanish Ministry of Economy and Competitiveness, ISCIII through CIBERer (Biomedical Network Research Center for Rare Diseases and INGENIO2010) and Prometeo PROMETEO/2018/135 Generalitat Valenciana to FVP. DP-C is a “Juan de la Cierva—Incorporación” fellow (grant number IJC2019-040237-I), Spanish Ministerio de Ciencia e Innovación.
Author information
Authors and Affiliations
Contributions
Experimental design: SN, CH, FVP, and JLGG.; experiment implementation: DP-C, CB-B, JLGG, and JSI-C; data analysis: DP-C, CB-B, JLGG, and JSI-C; writing paper: DP-C and SN. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Competing interests
JLGG and FVP have a patent granted related to a method based on mass spectrometry to quantify circulating histones in biological samples for the diagnosis of sepsis and septic shock. The other authors report no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1. Extracellular histone-induced reactive oxygen species production is mediated by COX and NOX activity.
2. COX/NOX-mediated increase in reactive oxygen species induced VCAM1 and ICAM1 expression through an NF-kB-dependent mechanism.
3. TLR4 is the main receptor involved in COX/NOX-mediated NF-kB/CAMs pathway activation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Cremades, D., Bueno-Betí, C., García-Giménez, J.L. et al. Extracellular histones trigger oxidative stress-dependent induction of the NF-kB/CAM pathway via TLR4 in endothelial cells. J Physiol Biochem 79, 251–260 (2023). https://doi.org/10.1007/s13105-022-00935-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00935-z