Abstract
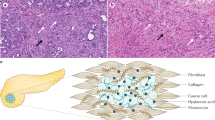
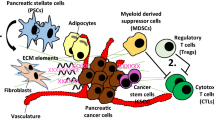
Pancreatic ductal adenocarcinoma (PDAC) is a type of cancer with limited treatment options and terrible long-term survival, and it is expected to become the second leading cause of cancer-related death by 2030. One reason why this cancer is so aggressive and resistant is the formation of dense stroma that surrounds the neoplastic epithelium, which promotes tumor progression, invasion, metastasis, and resistance. The three major components of PDAC stroma are extracellular matrix (ECM), cancer-associated fibroblasts (CAFs), and vasculature. The dense ECM acts as a natural physical barrier, impeding drug penetration to PDAC tumor cells. Consequently, the method that combines stroma-targeting with anticancer therapy may be a viable alternative for increasing drug penetration. Additionally, blood vessels are key entities of the tumor stroma, serving as a pathway for nutrition as well as the only way for chemical medicines and immune cells to act. Finally, PDAC CAFs and tumor cells have crosstalk effects in the tumor microenvironment, where they are responsible for enhanced matrix deposition. In this review, we aim to provide an overview of our current comprehension of the three key components of PDAC stroma and the new promising therapeutic targets for PDAC.

Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Aguilera KY, Huang H, Du W et al (2017) Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma. Mol Cancer Ther 16:2473–2485
Bachem MG, Schneider E, Groß H et al (1998) Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115:421–432
Bailey JM, Swanson BJ, Hamada T et al (2008) Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 14:5995–6004
Bernard V, Semaan A, Huang J et al (2019) Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Cancer Res 25:2194–2205
Biffi G, Oni TE, Spielman B et al (2019) IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 9:282–301
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15:786–801
Burris H 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Catenacci DV, Junttila MR, Karrison T et al (2015) Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 33:4284
Chauhan VP, Boucher Y, Ferrone CR et al (2014) Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26:14
Cheng X-B, Sato N, Kohi S, Yamaguchi K (2013) Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS ONE 8:e80765
Christian S, Pilch J, Akerman ME (2003) Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol 163:871–878
Ciardiello D, Elez E, Tabernero J, Seoane J (2020) Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann Oncol 31:1336–1349
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
De Jesus-Acosta A, O’Dwyer PJ, Ramanathan RK et al (2014) A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic pancreatic ductal adenocarcinoma (PDA). Br J Cancer 122(4):498–505
DuFort CC, DelGiorno KE, Carlson MA et al (2016) Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophys J 110:2106–2119
Dvorak HF (1986) Tumors: wounds that do not heal. N Engl J Med 315:1650–1659
Elyada E, Bolisetty M, Laise P et al (2019) Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 9:1102–1123
Erkan M, Adler G, Apte MV et al (2012) StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61:172–178
Feig C, Gopinathan A, Neesse A et al (2012) The pancreas cancer microenvironment. Clin Cancer Res 18:4266–4276
Fendrich V, Esni F, Garay MVR et al (2008) Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology 135:621–631
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285:1182–1186
Folkman J (1992) The role of angiogenesis in tumor growth. Seminars Semin Cancer Biol 3(2):65–71
Friess H, Yamanaka Y, Büchler M et al (1993) Enhanced expression of transforming growth factor β isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 105:1846–1856
Gaustad J-V, Simonsen TG, Wegner CS, Rofstad EK (2019) Vascularization, oxygenation, and the effect of Sunitinib treatment in pancreatic ductal adenocarcinoma Xenografts. Front Oncol 9:845
Gilles M-E, Maione F, Cossutta M et al (2016) Nucleolin targeting impairs the progression of pancreatic cancer and promotes the normalization of tumor vasculature. Can Res 76:7181–7193
Goel S, Duda DG, Xu L et al (2011) Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 91:1071–1121
Gore J, Korc M (2014) Pancreatic cancer stroma: friend or foe? Cancer Cell 25:711–712
Hamidi H, Ivaska J (2018) Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer 18:533–548
Hessmann E, Buchholz SM, Demir IE et al (2020) Microenvironmental determinants of pancreatic cancer. Physiol Rev 100:1707–1751
Hosein AN, Brekken RA, Maitra A (2020) Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol 17:487–505
Hovanessian AG, Soundaramourty C, Khoury DE et al (2010) Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS ONE 5:e15787
Jacobetz MA, Chan DS, Neesse A et al (2013) Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62:112–120
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7:987–989
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Jiang H, Hegde S, Knolhoff BL et al (2016) Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 22:851–860
Jiang H, Liu X, Knolhoff BL et al (2020) Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 69:122–132
Jones S, Zhang X, Parsons DW et al (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–1806
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16:582–598
Ko AH, LoConte N, Tempero MA et al (2016) A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 45:370
Kong F, Li L, Wang G et al (2019) VDR signaling inhibits cancer-associated-fibroblasts’ release of exosomal miR-10a-5p and limits their supportive effects on pancreatic cancer cells. Gut 68:950–951
Kongsbak M, von Essen MR, Boding L et al (2014) Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS ONE 9:e96695
Lee BY, Timpson P, Horvath LG, Daly RJ (2015) FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther 146:132–149
Li D, Xie K, Wolff R, Abbruzzese JL (2004) Pancreatic cancer. The Lancet 363:1049–1057
Li S, Xu H-X, Wu C-T et al (2019) Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis 22:15–36
Linder S, Castaños-Velez E, von Rosen A, Biberfeld P (2001) Immunohistochemical expression of extracellular matrix proteins and adhesion molecules in pancreatic carcinoma. Hepatogastroenterology 48:1321–1327
Liu H, Shi Y, Qian F (2021) Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv Drug Deliv Rev 172:37–51
Longo V, Brunetti O, Gnoni A et al (2016) Angiogenesis in pancreatic ductal adenocarcinoma: a controversial issue. Oncotarget 7:58649
Maione F, Molla F, Meda C et al (2009) Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Investig 119:3356–3372
Melisi D, Garcia-Carbonero R, Macarulla T et al (2018) Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer 119:1208–1214
Murphy KJ, Reed DA, Vennin C et al (2021) Intravital imaging technology guides FAK-mediated priming in pancreatic cancer precision medicine according to Merlin status. Science advances 7:eabh0363
Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
Nywening TM, Belt BA, Cullinan DR et al (2018) Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67:1112–1123
Öhlund D, Elyada E, Tuveson D (2014) Fibroblast heterogeneity in the cancer wound. J Exp Med 211:1503–1523
Öhlund D, Handly-Santana A, Biffi G et al (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214:579–596
Oldfield LE, Connor AA, Gallinger S (2017) Molecular events in the natural history of pancreatic cancer. Trends in cancer 3:336–346
Pereira BA, Vennin C, Papanicolaou M et al (2019) CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer 5:724–741
Principe DR, DeCant B, Mascariñas E et al (2016) TGFβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis. Can Res 76:2525–2539
Provenzano PP, Cuevas C, Chang AE et al (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429
Quante M, Tu SP, Tomita H et al (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272
Rath N, Morton JP, Julian L et al (2017) ROCK signaling promotes collagen remodeling to facilitate invasive pancreatic ductal adenocarcinoma tumor cell growth. EMBO Mol Med 9:198–218
Rath N, Olson MF (2012) Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep 13:900–908
Ruggeri JM, Franco-Barraza J, Sohail A et al (2020) Discoidin domain receptor 1 (DDR1) is necessary for tissue homeostasis in pancreatic injury and pathogenesis of pancreatic ductal adenocarcinoma. Am J Pathol 190:1735–1751
Sahai E, Astsaturov I, Cukierman E et al (2020) A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20:174–186
Serini G, Valdembri D, Zanivan S et al (2003) Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424:391–397
Sherman MH, Ruth TY, Engle DD et al (2014) Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159:80–93
Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR (2006) Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH2-terminal kinase 1 and up-regulating N-cadherin expression. Can Res 66:11745–11753
Siegel RL, Miller KD, Goding Sauer A et al (2020) Colorectal cancer statistics, 2020. CA: a Cancer J Clin 70:145–164
Strobel O, Neoptolemos J, Jäger D, Büchler MW (2019) Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 16:11–26
Sulzmaier FJ, Jean C, Schlaepfer DD (2014) FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 14:598–610
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J Clin 71:209–249
Thayer SP, di Magliano MP, Heiser PW et al (2003) Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425:851–856
Thomas D, Radhakrishnan P (2019) Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer 18:1–15
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4:528–539
Vaquero EC, Edderkaoui M, Nam KJ et al (2003) Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology 125:1188–1202
Vennin C, Chin VT, Warren SC et al (2017) Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med 9:eaai8504
Vennin C, Mélénec P, Rouet R et al (2019) CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun 10:1–22
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Waghray M, Yalamanchili M, Dziubinski M et al (2016) GM-CSF mediates mesenchymal–epithelial cross-talk in pancreatic cancer. Cancer Discov 6:886–899
Wallbaum P, Rohde S, Ehlers L et al (2018) Antifibrogenic effects of vitamin D derivatives on mouse pancreatic stellate cells. World J Gastroenterol 24:170
Wang-Gillam A (2019) Targeting stroma: a tale of caution. J Clin Oncol 37:1041–1043
Yang J, Zhang Y, He S et al (2017) TM4SF1 promotes metastasis of pancreatic cancer via regulating the expression of DDR1. Sci Rep 7:1–8
Zeisberg EM, Potenta S, Xie L et al (2007) Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Can Res 67:10123–10128
Zinger A, Koren L, Adir O et al (2019) Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano 13:11008–11021
Funding
This research was funded by by China Scholarship Council to XL; La Ligue Contre le Cancer, INCa, Canceropole PACA and INSERM to JI; INSERM to PSC.
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• PDAC is a deadly cancer characterized by a dense and extensive stromal matrix.
• The complex stromal components have brought great challenges to PDAC therapy.
• Stroma-targeting strategies will be prospective for the successful therapy of PDAC.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Iovanna, J. & Santofimia-Castaño, P. Stroma-targeting strategies in pancreatic cancer: a double-edged sword. J Physiol Biochem 79, 213–222 (2023). https://doi.org/10.1007/s13105-022-00941-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00941-1