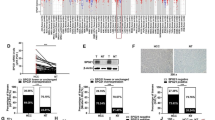
Abstract
Exosomes have a critical role in the intercellular communication and metastatic progression of hepatocellular carcinoma (HCC). Recently, our group showed that α2, 6-sialylation played an important role in the proliferation- and migration-promoting effects of cancer-derived exosomes. However, the molecular basis remains elusive. In this study, the mechanism of α2, 6-sialylation-mediated specific microRNAs (miRNA) sorting into exosomes was illustrated. We performed miRNA profiling analysis to compare exosomes from HCC cell lines that differ only in α2, 6-sialylation status. A total of 388 differentially distributed miRNAs were identified in wild-type and β-galactoside α2, 6-sialyltransferase I (ST6Gal-I) knockdown MHCC-97H cells-derived exosomes. Neutral sphingomyelinase-2 (nSmase2), an important regulator mediating the sorting of exosomal miRNAs, was found to be a target of ST6Gal-I. The reduction of α2, 6-sialylation could impair the activity of nSmase2, as well as the nSmase2-dependent exosomal miRNAs sorting. This α2,6-sialylation-dependent sorting exerted an augmentation of motility on recipient HCC cells. Our data further demonstrated that α2,6-sialylation-mediated sorting of exosomal miR-100-5p promoted the migration and invasion of recipient HepG2 cells via the PI3K/AKT signaling pathway. The cellular metastasis–related gene CLDN11 was confirmed as a direct target of exosomal miR-100-5p, which elevated the mobility of recipient HCC cells. In conclusion, our results showed that α2,6-sialylation modulates nSmase2-dependent exosomal miRNAs sorting and promotes HCC progression.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Büll C, Stoel MA, den Brok MH, Adema GJ (2014) Sialic acids sweeten a tumor’s life. Cancer Res 74:3199–3204
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D (2016) Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell 30:836–848
Britain CM, Holdbrooks AT, Anderson JC, Willey CD, Bellis SL (2018) Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J Ovarian Res 11:12
Chaiyawat P, Weeraphan C, Netsirisawan P, Chokchaichamnankit D, Srisomsap C, Svasti J, Champattanachai V (2016) Elevated O-GlcNAcylation of extracellular vesicle proteins derived from metastatic colorectal cancer cells. Cancer Genomics Proteomics 13:387–398
Chen L, Chen R, Kemper S, Brigstock DR (2018) Pathways of production and delivery of hepatocyte exosomes. J Cell Commun Signal 12:343–357
Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, Qi L, Liu Y, Wu Q, Cui Y, Fang F, Zhang X, Song T, Guo H (2018) HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis 9:513
Chen X, Wang L, Yu X, Wang S, Zhang J (2021) Caveolin-1 facilitates cell migration by upregulating nuclear receptor 4A2/retinoid X receptor α-mediated β-galactoside α2,6-sialyltransferase I expression in human hepatocarcinoma cells. Int J Biochem Cell Biol 137:106027
Chen X, Wang L, Zhao Y, Yuan S, Wu Q, Zhu X, Niang B, Wang S, Zhang J (2016) ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget 7:51955–51964
Ding Y, Mei W, Zheng Z, Cao F, Liang K, Jia Y, Wang Y, Liu D, Li J, Li F (2021) Exosomes secreted from human umbilical cord mesenchymal stem cells promote pancreatic ductal adenocarcinoma growth by transferring miR-100-5p. Tissue Cell 73:101623
Falcone G, Felsani A, D’Agnano I (2015) Signaling by exosomal microRNAs in cancer. J Exp Clin Cancer Res 34:32
Ge Y, Mu W, Ba Q, Li J, Jiang Y, Xia Q, Wang H (2020) Hepatocellular carcinoma-derived exosomes in organotropic metastasis, recurrence and early diagnosis application. Cancer Lett 477:41–48
Ghosh S, Bhowmik S, Majumdar S, Goswami A, Chakraborty J, Gupta S, Aggarwal S, Ray S, Chatterjee R, Bhattacharyya S, Dutta M, Datta S, Chowdhury A, Dhali GK, Banerjee S (2020) The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int J Cancer 147:2934–2947
Groot M, Lee H (2020) Sorting mechanisms for microRNAs into extracellular vesicles and their associated diseases. Cells 9:1044
Huang H, Liu Y, Yu P, Qu J, Guo Y, Li W, Wang S, Zhang J (2018) MiR-23a transcriptional activated by Runx2 increases metastatic potential of mouse hepatoma cell via directly targeting Mgat3. Sci Rep 8:7366
Jung YR, Park JJ, Jin YB, Cao YJ, Park MJ, Kim EJ, Lee M (2016) Silencing of ST6Gal I enhances colorectal cancer metastasis by down-regulating KAI1 via exosome-mediated exportation and thereby rescues integrin signaling. Carcinogenesis 37:1089–1097
Kai K, Dittmar RL, Sen S (2018) Secretory microRNAs as biomarkers of cancer. Semin Cell Dev Biol 78:22–36
Karaman B, Battal B, Sari S, Verim S (2014) Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 20:18059–18060
Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T (2013) Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem 288:10849–10859
Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285:17442–17452
Liang Y, Eng WS, Colquhoun DR, Dinglasan RR, Graham DR, Mahal LK (2014) Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J Biol Chem 289:32526–32537
Lin M, Liao W, Dong M, Zhu R, Xiao J, Sun T, Chen Z, Wu B, Jin J (2018) Exosomal neutral sphingomyelinase 1 suppresses hepatocellular carcinoma via decreasing the ratio of sphingomyelin/ceramide. Febs j 285:3835–3848
Lin Q, Zhou CR, Bai MJ, Zhu D, Chen JW, Wang HF, Li MA, Wu C, Li ZR, Huang MS (2020) Exosome-mediated miRNA delivery promotes liver cancer EMT and metastasis. Am J Transl Res 12:1080–1095
Menck K, Scharf C, Bleckmann A, Dyck L, Rost U, Wenzel D, Dhople VM, Siam L, Pukrop T, Binder C, Klemm F (2015) Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J Mol Cell Biol 7:143–153
Pan JH, Zhou H, Zhao XX, Ding H, Li W, Qin L, Pan YL (2018) Role of exosomes and exosomal microRNAs in hepatocellular carcinoma: potential in diagnosis and antitumour treatments (Review). Int J Mol Med 41:1809–1816
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18:883–891
Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y (2016) Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res 35:159
Rahmani F, Ziaeemehr A, Shahidsales S, Gharib M, Khazaei M, Ferns GA, Ryzhikov M, Avan A, Hassanian SM (2020) Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. J Cell Physiol 235:4146–4152
Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S, Moriyama M (2019) Exosomes and hepatocellular carcinoma: from bench to bedside. Int J Mol Sci 20:1406
Singh R, Pochampally R, Watabe K, Lu Z, Mo YY (2014) Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer 13:256
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Tan W, Li Z, Xia W, Zhu J, Fan R (2022) miR-221-3p regulates hepatocellular carcinoma cell proliferation, migration and invasion via targeting LIFR. Ann Hepatol 27(Suppl 1):100567
Tan Z, Cao L, Wu Y, Wang B, Song Z, Yang J, Cheng L, Yang X, Zhou X, Dai Z, Li X, Guan F (2020) Bisecting GlcNAc modification diminishes the pro-metastatic functions of small extracellular vesicles from breast cancer cells. J Extracell Vesicles 10:e12005
Wang L, Chen X, Wang L, Wang S, Li W, Liu Y, Zhang J (2021) Knockdown of ST6Gal-I expression in human hepatocellular carcinoma cells inhibits their exosome-mediated proliferation- and migration-promoting effects. IUBMB Life 73:1378–1391
Wang L, Li S, Yu X, Han Y, Wu Y, Wang S, Chen X, Zhang J, Wang S (2019) α2,6-Sialylation promotes immune escape in hepatocarcinoma cells by regulating T cell functions and CD147/MMP signaling. J Physiol Biochem 75:199–207
Williams C, Pazos R, Royo F, González E, Roura-Ferrer M, Martinez A, Gamiz J, Reichardt NC, Falcón-Pérez JM (2019) Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci Rep 9:11920
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ (2018) Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 15:617–638
Yang G, Jian L, Chen Q (2021) Comprehensive analysis of expression and prognostic value of the claudin family in human breast cancer. Aging (Albany NY) 13:8777–8796
Yang J, Liu X, Yuan X, Wang Z (2015) miR-99b promotes metastasis of hepatocellular carcinoma through inhibition of claudin 11 expression and may serve as a prognostic marker. Oncol Rep 34:1415–1423
Yuan Q, Chen X, Han Y, Lei T, Wu Q, Yu X, Wang L, Fan Z, Wang S (2018) Modification of α2,6-sialylation mediates the invasiveness and tumorigenicity of non-small cell lung cancer cells in vitro and in vivo via Notch1/Hes1/MMPs pathway. Int J Cancer 143:2319–2330
Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics 13:17–24
Zhang XZ, Mao HL, Zhang SJ, Sun L, Zhang WJ, Chen QZ, Wang L, Liu HC (2020) lncRNA PCAT18 inhibits proliferation, migration and invasion of gastric cancer cells through miR-135b suppression to promote CLDN11 expression. Life Sci 249:117478
Zheng J, Guo X, Gao X, Liu H, Tu Y, Zhang Y (2014) Overexpression of retinoic acid-induced protein 3 predicts poor prognosis for hepatocellular carcinoma. Clin Transl Oncol 16:57–63
Funding
This study was supported by the National Natural Science Foundation of China (31971214, 32171282, 31870793) and the Fundamental Research Funds for the Central Universities (DUT20YG116 and DUT20YG130).
Author information
Authors and Affiliations
Contributions
Liping Wang, Jianing Zhang, and Yubo Liu conceived and designed the study. Liping Wang, Xixi Chen, Fanxu Meng, Tianmiao Huang, Shujing Wang, Zhichao Zheng, Guoliang Zheng performed the experiments. Liping Wang and Liu Yubo wrote the paper. Wenli Li and Jianing Zhang reviewed and edited the manuscript. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• ST6Gal-I silencing affects the release of exosomal miRNAs from HCC cells.
• nSmase2 is a target for ST6Gal-I α2,6-sialylation.
• α2,6-sialylation regulates exosomal sorting by interfering with nSmase2 activity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Chen, X., Meng, F. et al. α2,6-Sialylation promotes hepatocellular carcinoma cells migration and invasion via enhancement of nSmase2-mediated exosomal miRNA sorting. J Physiol Biochem 79, 19–34 (2023). https://doi.org/10.1007/s13105-022-00917-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00917-1