Abstract
Background
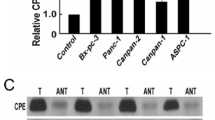
Metastasis-related in colon cancer 1 (MACC1) is highly expressed in a variety of solid tumours, but its role in pancreatic cancer (PC) remains unknown. Interferon gamma (IFN-γ) affecting MACC1 expression was explored as the potential mechanism following its intervention.
Methods
Expressions of MACC1 treated with IFN-γ gradient were confirmed by quantitative real-time PCR (qRT-PCR) and western blot (WB). Proliferation, migration, and invasion abilities of PC cells treated with IFN-γ were analysed by CCK8, EDU, colony formation, Transwell (with or without matrix gel) and wound-healing assays. Expression of antisense long non-coding RNA of MACC1, MACC1-AS1, and proteins of AKT/mTOR pathway, (pho-)AKT, and (pho-)mTOR was also assessed by qRT-PCR and WB. SiRNA kit and lentiviral fluid were conducted for transient expression of MACC1 and stable expression of MACC1-AS1, respectively. Rescue assays of cells overexpressing MACC1-AS1 and of cells silencing MACC1 were performed and cellular properties and proteins were assessed by the above-mentioned assays as well.
Results
IFN-γ inhibited MACC1 expression in a time- and dose-dependent manner; 100 ng/mL IFN-γ generally caused downregulation of most significant (p ≤ 0.05). In vitro experiments revealed that IFN-γ decreased cellular proliferation, migration, and invasion abilities and downregulated the expression of pho-AKT and pho-mTOR (p ≤ 0.05). Conversely, overexpression of MACC1-AS1 upregulated pho-AKT and pho-mTOR proteins, and reversed cellular properties (p ≤ 0.05). Rescue assays alleviated the above changes of pho-AKT/ mTOR and cellular properties.
Conclusion
IFN-γ affected PC properties by MACC1-AS1/MACC1 axis via AKT/mTOR signaling pathway, which provides novel insight for candidate targets for treating PC.







Similar content being viewed by others
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Pai RK, Pai RK. Pathologic assessment of gastrointestinal tract and pancreatic carcinoma after neoadjuvant therapy. Mod Pathol. 2018;31(1):4–23. https://doi.org/10.1038/modpathol.2017.87.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Montejo Gañán I, Ángel Ríos LF, Sarría Octavio de Toledo L, Martínez Mombila ME, Ros Mendoza LH. Staging pancreatic carcinoma by computed tomography. Radiologia (Engl Ed). 2018;60(1):10–23.https://doi.org/10.1016/j.rx.2017.08.004.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
Ferrantini M, Capone I, Belardelli F. Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie. 2007;89(6–7):884–93. https://doi.org/10.1016/j.biochi.2007.04.006.
Ni C, Wu P, Zhu X, Ye J, Zhang Z, Chen Z, et al. IFN-γ selectively exerts pro-apoptotic effects on tumor-initiating label-retaining colon cancer cells. Cancer Lett. 2013;336(1):174–84. https://doi.org/10.1016/j.canlet.2013.04.029.
Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-γ mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol. 2013;182(6):2345–54. https://doi.org/10.1016/j.ajpath.2013.02.041.
Kammertoens T, Friese C, Arina A, Idel C, Briesemeister D, Rothe M, et al. Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature. 2017;545(7652):98–102. https://doi.org/10.1038/nature22311.
Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in Tumor Microenvironment. Comput Struct Biotechnol J. 2019;17:1–13. https://doi.org/10.1016/j.csbj.2018.11.004.
Tau GZ, Cowan SN, Weisburg J, Braunstein NS, Rothman PB. Regulation of IFN-gamma signaling is essential for the cytotoxic activity of CD8(+) T cells. J Immunol. 2001;167(10):5574–82. https://doi.org/10.4049/jimmunol.167.10.5574.
Mojic M, Takeda K, Hayakawa Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int J Mol Sci. 2017;19(1). https://doi.org/10.3390/ijms19010089.
Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, et al. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. 2015;16(1):64. https://doi.org/10.1186/s13059-015-0620-6.
Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-γ links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469(7331):548–53. https://doi.org/10.1038/nature09666.
Zhang Y, Wang Z, Chen M, Peng L, Wang X, Ma Q, et al. MicroRNA-143 targets MACC1 to inhibit cell invasion and migration in colorectal cancer. Mol Cancer. 2012;11:23. https://doi.org/10.1186/1476-4598-11-23.
Huang Y, Zhang H, Cai J, Fang L, Wu J, Ye C, et al. Overexpression of MACC1 and Its significance in human Breast Cancer Progression. Cell Biosci. 2013;3(1):16. https://doi.org/10.1186/2045-3701-3-16.
Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med. 2009;15(1):59–67. https://doi.org/10.1038/nm.1889.
Lemos C, Hardt MS, Juneja M, Voss C, Förster S, Jerchow B, et al. MACC1 induces tumor progression in transgenic mice and colorectal cancer patients via increased pluripotency markers nanog and Oct4. Clin Cancer Res. 2016;22(11):2812–24. https://doi.org/10.1158/1078-0432.Ccr-15-1425.
Galimi F, Torti D, Sassi F, Isella C, Corà D, Gastaldi S, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res. 2011;17(10):3146–56. https://doi.org/10.1158/1078-0432.Ccr-10-3377.
Stein U. MACC1 - a novel target for solid cancers. Expert Opin Ther Targets. 2013;17(9):1039–52. https://doi.org/10.1517/14728222.2013.815727.
Hagemann C, Fuchs S, Monoranu CM, Herrmann P, Smith J, Hohmann T, et al. Impact of MACC1 on human malignant glioma progression and patients’ unfavorable prognosis. Neuro Oncol. 2013;15(12):1696–709. https://doi.org/10.1093/neuonc/not136.
Shimokawa H, Uramoto H, Onitsuka T, Chundong G, Hanagiri T, Oyama T, et al. Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg. 2011;141(4):895–8. https://doi.org/10.1016/j.jtcvs.2010.09.044.
Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17(19):6118–24. https://doi.org/10.1158/1078-0432.Ccr-11-0482.
Kobelt D, Zhang C, Clayton-Lucey IA, Glauben R, Voss C, Siegmund B, et al. Pro-inflammatory TNF-α and IFN-γ promote tumor growth and metastasis via Induction of MACC1. Front Immunol. 2020;11:980. https://doi.org/10.3389/fimmu.2020.00980.
Pan-Cancer Analysis Pinpoints Targets in PI3K Pathway. Cancer Discov. 2017;7(8):Of6.https://doi.org/10.1158/2159-8290.Cd-nb2017-092.
Zhang Y, Kwok-Shing Ng P, Kucherlapati M, Chen F, Liu Y, Tsang YH, et al. A Pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell. 2017;31(6):820-32.e3. https://doi.org/10.1016/j.ccell.2017.04.013.
Zheng D, Che D, Lin F, Wang X, Lu L, Chen J, et al. LncRNA MACC1-AS1/MACC1 enhances the progression of glioma via regulating metabolic plasticity. Cell Cycle. 2020;19(18):2286–97. https://doi.org/10.1080/15384101.2020.1795595.
Tong G, Cheng B, Li J, Wu X, Nong Q, He L, et al. MACC1 regulates PDL1 expression and tumor immunity through the c-Met/AKT/mTOR pathway in gastric cancer cells. Cancer Med. 2019;8(16):7044–54. https://doi.org/10.1002/cam4.2542.
Zhang YM, Wu QM, Chang LY, Liu JC. miR-34a and miR-125a-5p inhibit proliferation and metastasis but induce apoptosis in hepatocellular carcinoma cells via repressing the MACC1-mediated PI3K/AKT/mTOR pathway. Neoplasma. 2020;67(5):1042–53. https://doi.org/10.4149/neo_2020_191019N1062.
Zhou W, Liu L, Xue Y, Zheng J, Liu X, Ma J, et al. Combination of endothelial-monocyte-activating polypeptide-II with temozolomide suppress malignant biological behaviors of human glioblastoma stem cells via miR-590–3p/MACC1 Inhibiting PI3K/AKT/mTOR signal pathway. Front Mol Neurosci. 2017;10:68. https://doi.org/10.3389/fnmol.2017.00068.
Radhakrishnan H, Walther W, Zincke F, Kobelt D, Imbastari F, Erdem M, et al. MACC1-the first decade of a key metastasis molecule from gene discovery to clinical translation. Cancer Metastasis Rev. 2018;37(4):805–20. https://doi.org/10.1007/s10555-018-9771-8.
Muendlein A, Hubalek M, Geller-Rhomberg S, Gasser K, Winder T, Drexel H, et al. Significant survival impact of MACC1 polymorphisms in HER2 positive breast cancer patients. Eur J Cancer. 2014;50(12):2134–41. https://doi.org/10.1016/j.ejca.2014.05.007.
Guo L, Ou S, Ma X, Zhang S, Lai Y. MACC1 silencing inhibits cell proliferation and induces cell apoptosis of lung adenocarcinoma cells through the β-catenin pathway. Neoplasma. 2018;65(4):552–60. https://doi.org/10.4149/neo_2018_170918N595.
Li Z, Yanfang W, Li J, Jiang P, Peng T, Chen K, et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–50. https://doi.org/10.1016/j.canlet.2018.04.035.
Yu L, Mao X, Jiao Y, Song W, Wang D. Expression of vasohibin-1, MACC1 and KA11 proteins in serous ovarian cancer and their clinical significance. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2019;44(12):1344–52. https://doi.org/10.11817/j.issn.1672-7347.2019.180391.
Chen XP, Ren XP, Lan JY, Chen YG, Shen ZJ. Analysis of HGF, MACC1, C-met and apoptosis-related genes in cervical carcinoma mice. Mol Biol Rep. 2014;41(3):1247–56. https://doi.org/10.1007/s11033-013-2969-5.
Yao Y, Dou C, Lu Z, Zheng X, Liu Q. MACC1 suppresses cell apoptosis in hepatocellular carcinoma by targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem. 2015;35(3):983–96. https://doi.org/10.1159/000369754.
Liu J, Pan C, Guo L, Wu M, Guo J, Peng S, et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the Warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9(1):76. https://doi.org/10.1186/s13045-016-0302-1.
Zhen T, Dai S, Li H, Yang Y, Kang L, Shi H, et al. MACC1 promotes carcinogenesis of colorectal cancer via β-catenin signaling pathway. Oncotarget. 2014;5(11):3756–69. https://doi.org/10.18632/oncotarget.1993.
Wang G, Kang MX, Lu WJ, Chen Y, Zhang B, Wu YL. MACC1: a potential molecule associated with pancreatic cancer metastasis and chemoresistance. Oncol Lett. 2012;4(4):783–91. https://doi.org/10.3892/ol.2012.784.
Wang L, Zhou R, Zhao Y, Dong S, Zhang J, Luo Y, et al. MACC-1 promotes endothelium-dependent angiogenesis in gastric cancer by activating TWIST1/VEGF-a signal pathway. PLoS ONE. 2016;11(6):e0157137. https://doi.org/10.1371/journal.pone.0157137.
Dijkmans R, Billiau A. Interferon gamma: a master key in the immune system. Curr Opin Immunol. 1988;1(2):269–74. https://doi.org/10.1016/0952-7915(88)90013-1.
Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1(6):447–56. https://doi.org/10.1016/1074-7613(94)90087-6.
Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410(6832):1107–11. https://doi.org/10.1038/35074122.
Wang L, Wang Y, Song Z, Chu J, Qu X. Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. J Interferon Cytokine Res. 2015;35(4):273–80. https://doi.org/10.1089/jir.2014.0132.
Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95(13):7556–61. https://doi.org/10.1073/pnas.95.13.7556.
George J, Banik NL, Ray SK. Knockdown of hTERT and concurrent treatment with interferon-gamma inhibited proliferation and invasion of human glioblastoma cell lines. Int J Biochem Cell Biol. 2010;42(7):1164–73. https://doi.org/10.1016/j.biocel.2010.04.002.
Zhao YH, Wang T, Yu GF, Zhuang DM, Zhang Z, Zhang HX, et al. Anti-proliferation effects of interferon-gamma on gastric cancer cells. Asian Pac J Cancer Prev. 2013;14(9):5513–8. https://doi.org/10.7314/apjcp.2013.14.9.5513.
Wang XY, Crowston JG, White AJ, Zoellner H, Healey PR. Interferon-alpha and interferon-gamma modulate Fas-mediated apoptosis in mitomycin-C-resistant human Tenon’s fibroblasts. Clin Exp Ophthalmol. 2014;42(6):529–38. https://doi.org/10.1111/ceo.12268.
Yuan L, Zhou C, Lu Y, Hong M, Zhang Z, Zhang Z, et al. IFN-γ-mediated IRF1/miR-29b feedback loop suppresses colorectal cancer cell growth and metastasis by repressing IGF1. Cancer Lett. 2015;359(1):136–47. https://doi.org/10.1016/j.canlet.2015.01.003.
Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97(1):192–7. https://doi.org/10.1182/blood.v97.1.192.
Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, et al. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med. 2006;203(6):1391–7. https://doi.org/10.1084/jem.20060436.
Matsuda M, Nakamoto Y, Suzuki S, Kurata T, Kaneko S. Interferon-gamma-mediated hepatocarcinogenesis in mice treated with diethylnitrosamine. Lab Invest. 2005;85(5):655–63. https://doi.org/10.1038/labinvest.3700257.
Xiao M, Wang C, Zhang J, Li Z, Zhao X, Qin Z. IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res. 2009;69(5):2010–7. https://doi.org/10.1158/0008-5472.Can-08-3479.
Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–87. https://doi.org/10.1038/onc.2011.621.
Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. https://doi.org/10.1016/j.cell.2013.06.020.
Qi C, Xiaofeng C, Dongen L, Liang Y, Liping X, Yue H, et al. Long non-coding RNA MACC1-AS1 promoted pancreatic carcinoma progression through activation of PAX8/NOTCH1 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):344. https://doi.org/10.1186/s13046-019-1332-7.
He W, Liang B, Wang C, Li S, Zhao Y, Huang Q, et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38(23):4637–54. https://doi.org/10.1038/s41388-019-0747-0.
Zhao Y, Liu Y, Lin L, Huang Q, He W, Zhang S, et al. The lncRNA MACC1-AS1 promotes gastric cancer cell metabolic plasticity via AMPK/Lin28 mediated mRNA stability of MACC1. Mol Cancer. 2018;17(1):69. https://doi.org/10.1186/s12943-018-0820-2.
Hung CM, Kuo DH, Chou CH, Su YC, Ho CT, Way TD. Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells. J Agric Food Chem. 2011;59(17):9683–90. https://doi.org/10.1021/jf2021489.
Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29(36):4989–5005. https://doi.org/10.1038/onc.2010.236.
Acknowledgements
The authors thank for the help of Key laboratory of High-Incidence-Tumor Prevention & Treatment (Guangxi Medical University) and Research Institute of Innovative Think-tank in Guangxi Medical University (The gene–environment interaction in hepatocarcinogenesis in Guangxi HCCs and its translational applications in the HCC prevention).
Funding
This work has been financially supported by the National Natural Science Foundation of China (Grant Nos. 31560257 and 81960439), the “139” Plan for Training High Level Cadre Talents in Guangxi Medicine (G201903004).
Author information
Authors and Affiliations
Contributions
X-YS, and S-Y Qin designed this study. X-YS wrote this manuscript and S-Y Qin guided the revision of the manuscript and submitted for publication. All of the authors conducted the study, and approved the final report.
Corresponding author
Ethics declarations
Conflict of interest
The authors report there is no conflict of interest.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, XY., Zhang, XL., Shi, QY. et al. IFN-γ affects pancreatic cancer properties by MACC1-AS1/MACC1 axis via AKT/mTOR signaling pathway. Clin Transl Oncol 24, 1073–1085 (2022). https://doi.org/10.1007/s12094-021-02748-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02748-w