Abstract
Purpose
To review current measures for renal cancer care and develop a comprehensive and updated list of measures for their practical use in Spain.
Methods
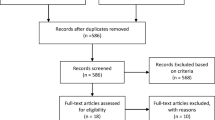
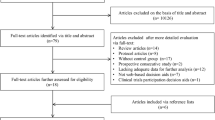
The study was developed by Fundación ECO, a Spanish foundation aiming to improve oncology quality of care. A systematic literature review was carried out to identify measures and knowledge gaps. A scientific committee composed of nine experts reviewed the literature findings and added measures. A preliminary list of 42 measures was evaluated with the Delphi method to gather feedback from 47 medical oncology experts in Spain. Experts scored the appropriateness of the measures and ranked their priority in two consecutive online surveys. The scientific committee reviewed the Delphi results and developed the measures. A technical group from Universidad Francisco de Vitoria conducted and oversaw the Delphi method.
Results
The Delphi method led to consensus on all 42 measures. The scientific committee used a prioritisation matrix to select 25 of these measures for evaluating quality of care in renal cancer. These measures regarded structure, process, and outcome and covered general management, diagnosis, treatment, follow-up, and evaluation of health outcomes. Easy-to-use index cards were developed for all 25 measures, including their definition, formula, acceptable level of attainment, and rationale.
Conclusions
This manuscript aims to provide healthcare professionals with expert- and evidence-based measures that are useful for evaluating quality of care in renal cancer in Spain and cover all aspects and stages.


Similar content being viewed by others
Change history
17 April 2022
The original online version of this article was revised: to include the supplementary material.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer observatory: cancer today. 2020. https://gco.iarc.fr/today. Accessed 10 Mar 2021.
Surveillance epidemiology and end results database. Cancer of the kidney and renal pelvis—SEER 18, relative survival by stage (2010–2017). 2021.
Karanikolos M, Ellis L, Coleman MP, McKee M. Health systems performance and cancer outcomes. J Natl Cancer Inst Monogr. 2013;46:7–12.
Chiang AC. Why the quality oncology practice initiative matters: it’s not just about cost. Am Soc Clin Oncol Educ B. 2016;36:e102–7.
Lawson KA, Saarela O, Abouassaly R, Kim SP, Breau RH, Finelli A. The impact of quality variations on patients undergoing surgery for renal cell carcinoma: a national cancer database study. Eur Urol. 2017;72(3):379–86.
Peabody H, Patel A, Johnson A, Mirza M, Noyes SL, Schervish E, et al. Development of a novel scoring system quantifies opportunities to reduce surgery for benign renal neoplasms: a retrospective quality improvement analysis within the MUSIC-KIDNEY Collaborative. J Urol. 2020;204(6):1160–5.
Johnson K, Lane BR, Weizer AZ, Herrel LA, Rogers CG, Qi J, et al. Partial nephrectomy should be classified as an inpatient procedure: results from a statewide quality improvement collaborative. Urol Oncol Semin Orig Investig. 2021;S1078–1439(21):00001–6.
Moideen N, Marzouk KH, Matheson KJ, Wood LA. Measuring quality care in localized renal cell cancer: use of appropriate preoperative investigations in a population-based cohort. Curr Oncol. 2017;24(2):e152–6.
Scottish Cancer Taksforce National Cancer Quality Steering Group. Renal cancer clinical quality performance indicators (v3.1). 2017.
Wood L, Bjarnason GA, Black PC, Cagiannos I, Heng DYC, Kapoor A, et al. Using the delphi technique to improve clinical outcomes through the development of quality indicators in renal cell carcinoma. J Oncol Pract. 2013. https://doi.org/10.1200/JOP.2012.000870.
American Society of Clinical Oncology. Quality Oncology Practice Initiative (QOPI®). 2021. https://practice.asco.org/quality-improvement/quality-programs/quality-oncology-practice-initiative/qopi-related-measures. Accessed 10 Mar 2021.
Sociedad Española de Calidad Asistencial. Indicadores de calidad para hospitales del sistema nacional de salud [Quality measures for hospitals belonging to the national public health system]. 2012 http://www.calidadasistencial.es/images/gestion/biblioteca/335.pdf.
Fitch K, J BS, Aguilar MD, Burnand B, LaCalle JR, Lazaro P, et al. The RAND/UCLA appropriateness method user’s manual. 2001.
Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151(9):1825–32.
Asociación Española de Urología. Indicadores de Calidad Asistencial en Urología. 2011. https://www.aeu.es/userfiles/indicadoresdecalidadenurologia.pdf.
D’Amico TA, Bandini LAM, Balch A, Benson AB, Edge SB, Lyn Fitzgerald C, et al. Quality measurement in cancer care: a review and endorsement of high-impact measures and concepts. JNCCN J Natl Compr Cancer Netw. 2020;18(3):250–9.
Stewart K, Bray B, Buckingham R. Improving quality of care through national clinical audit. Futur Hosp J. 2016;3(3):203–6.
Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD000259.pub3.
Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321.
Brown B, Gude WT, Blakeman T, Van Der Veer SN, Ivers N, Francis JJ, et al. Clinical performance feedback intervention theory (CP-FIT): a new theory for designing, implementing, and evaluating feedback in health care based on a systematic review and meta-synthesis of qualitative research. Implement Sci. 2019. https://doi.org/10.1186/s13012-019-0883-5.
World Health Organization. Regional Office for Europe EO on HS and P, Busse R, Klazinga N, Panteli D, Quentin W. Improving healthcare quality in Europe: Characteristics, effectiveness and implementation of different strategies. Improv Healthc Qual Eur. 2019.
Spanish Ministry of Health Consumer Affairs and Social Welfare. Healthcare systems in the European Union countries—Health characteristics and indicators. 2019 https://www.mscbs.gob.es/estadEstudios/estadisticas/docs/presentacion_en.pdf.
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE. 2011. https://doi.org/10.1371/journal.pone.0020476.
Acknowledgements
The authors thank the experts who participated in the Delphi study. The authors also thank Angela Rynne Vidal, PhD, for providing medical writing services, and Irene Santamaría Rodríguez from Universidad Francisco Vitoria for providing project support.
Funding
This study was funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
María José Méndez Vidal has received honoraria for honoraria for speaker and advisory role from Astellas, Astra Zeneca, Janssen, MSD, Bayer, Pfizer, Eisai, Ipsen, Sanofi, Roche, and BMS. Miguel Ángel Climent Durán has received honoraria for speaker engagements or advisory roles from BMS, Astellas, Janssen, MSD, Sanofi, Bayer, Roche, Pfizer, EUNSA, Pierre Fabre, Novartis and Ipsen; and travel funding from Janssen, Astellas, Roche, Ipsen, and MSD. Enrique Gallardo has received honoraria for speaker engagements, advisory roles or continuous medical education from Sanofi, Janssen, Astellas, Bayer, Ipsen, Pfizer, Roche, Novartis, Eisai, EUSA Pharma, BMS, AstraZeneca, Merck, Rovi, Daiichi Sankyo, Techdow, Rovi, Leo Pharma, Menarini, and Boehringer-Ingelheim; consulting fees from Merck and EUSA Pharma; and grant support from Astellas, Janssen, Sanofi, Bayer, Ipsen, Ferrer, Pfizer, Roche, GSK, BMS. Aránzazu González del Alba has received honoraria for speaker engagements, advisory roles or continuous medical education from Astellas, Sanofi, Astra Zeneca, Eusa Pharma, Janssen, Bayer, Pfizer, Novartis, Eisai, Ipsen, Roche, BMS, and Pierre Fabre; research funding from Astellas; and consulting fees from Astellas, Janssen. Martín Lázaro-Quintela reports honoraria for speaker engagements, advisory roles or continuous medical education from Roche, Eisai, Astellas, Janssen, Astra-Zeneca, MSD, Bayer, Pfizer, Eusa, Ipsen, Takeda, Sanofi, Roche, BMS, Merck, and Boehringer-Ingelheim; and research funding from Roche, Pfizer, and Janssen. Álvaro Pinto has received honoraria for advisory roles or participation in clinical trials from Pfizer, Novartis, Ipsen, BMS, Janssen, Astellas, Sanofi, Bayer, Clovis, Pharmacyclics, Astra Zeneca, Eisai, Roche, MSD, Pierre Fabre and Merck; research funding from Pfizer and BMS; and travel funding from Janssen, Roche, Pfizer, BMS, and Ipsen. Javier Puente has received honoraria for speaker engagements, advisory roles, or continuous medical education from Astellas, Astra Zeneca, Janssen, MSD, Bayer, Pfizer, Eisai, Ipsen, Sanofi, Roche, BMS, Pierre Fabre, Merck; research funding from Astellas and Pfizer; and consulting fees from Astellas and Roche. The other authors have no conflicts of interest to declare.
Ethical approval
This study did not involve humans and did not require approval from an Ethical Committee.
Informed consent
This study did not involve humans and informed consent was not necessary.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guillem Porta, V., Camps, C., Climent Durán, M.Á. et al. Measures to evaluate quality of care in renal cancer: results of a Delphi study in Spain. Clin Transl Oncol 24, 495–502 (2022). https://doi.org/10.1007/s12094-021-02703-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02703-9