Abstract
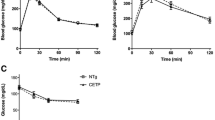
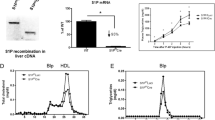
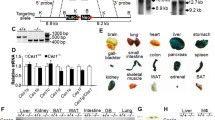
Cholesteryl ester transfer protein (CETP) and phospholipid transfer protein (PLTP) belong to the same gene family. Liver-specific expression of CETP improves reverse cholesterol transport (RCT) and PLTP knockout (KO) decreases RCT in mice. In this study, we investigate the effect of CETP transgene (CETP-tg) on RCT and whether CETP-tg can partially restore RCT efficiency in PLTP KO mice. Several rounds of crossing were carried out to produce colonies of wild type (WT), CETP-tg, PLTP KO, and CETP-tg × PLTP KO mice were obtained after several generations of reproduction. The efficiency of RCT was detected using [3H]-cholesterol-laden macrophages, and the underlying mechanisms were investigated by multiple techniques. Our data demonstrated that CETP-tg significantly increased the transport rate of [3H]-cholesterol from macrophages to plasma and liver, and finally the excretion through feces compared to the WT littermates. The RCT improving effect of CETP-tg was similar in PLTPKO mice. Furthermore, CETP-tg did not affect the expression of RCT-related proteins, such as low-density lipoprotein receptor. The mechanisms of improving RCT may be attributed to the low level of oxidized lipids in CETP-tg mouse and CETP-mediated lipid transport. Collectively, CETP-tg improves RCT in mice, and CETP can not compensate for PLTP deficiency.





Similar content being viewed by others
References
Agellon LB, Walsh A, Hayek T, Moulin P, Jiang XC, Shelanski SA, Breslow JL, Tall AR (1991) Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem 266(17):10796–10801
Atger V, de la Llera MM, Bamberger M, Francone O, Cosgrove P, Tall A, Walsh A, Moatti N, Rothblat G (1995) Cholesterol efflux potential of sera from mice expressing human cholesteryl ester transfer protein and/or human apolipoprotein AI. J Clin Invest 96(6):2613–2622. https://doi.org/10.1172/JCI118326
Barter P (2009) Lessons learned from the investigation of lipid level management to understand its impact in atherosclerotic events (ILLUMINATE) trial. Am J Cardiol 104(10 Suppl):10E-E15. https://doi.org/10.1016/j.amjcard.2009.09.014
Bell TA 3rd, Graham MJ, Lee RG, Mullick AE, Fu WX, Norris D, Crooke RM (2013) Antisense oligonucleotide inhibition of cholesteryl ester transfer protein enhances reverse cholesterol transport in hyperlipidemic CETP transgenic LDL-/- mice. J Lipid Res 54(10):2647–2657. https://doi.org/10.1194/jlr.M036509
Bighetti EJB, Patrício PR, Casquero AC, Berti JA, Oliveira HCF (2009) Ciprofibrate increases cholesteryl ester transfer protein gene expression and the indirect reverse cholesterol transport to liver. Lipids Health Dis 8:50. https://doi.org/10.1186/1476-511X-8-50
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Brown ML, Inazu A, Hesler CB, Agellon LB, Mann C, Whitlock ME, Marcel YL, Milne RW, Koizumi J, Mabuchi H (1989) Molecular basis of loipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature 342(6248):448–451. https://doi.org/10.1038/342448a0
Bruce C, Chouinard RA, Tall AR (1998) Plasma lipid transfer protein, high-density lipoproteins, and reverse cholesterol transport. Annu Rev Nutr 18:297–330. https://doi.org/10.1146/annurev.nutr.18.1.297
Chirasani VR, Revanasiddappa PD, Senapati S (2016) Structural plasticity of cholesteryl ester transfer protein assists the lipid transfer activity. J Biol Chem 291(37):19462–19473. https://doi.org/10.1074/jbc.M116.744623
Francone OL, Royer L, Haghpassand M (1996) Increased preβ-HDL levels, cholesterol efflux, and LCAT-mediated esterification in mice expressing the human cholesteryl ester transfer protein (CETP) and human apolipoprotein A-I (apoA-I) transgenes. J Lipid Res 37(6):1268–1277
Gordon DJ, Rifkind BM (1989) High density lipoprotein: the clinical implications of recent studies. N Engl J Med 321(19):1311–1316. https://doi.org/10.1056/NEJM198911093211907
Harder C, Lau P, Meng A, Whitman SC, McPherson R (2007) Cholesteryl ester transfer protein (CETP) expression protects against diet induced atherosclerosis in SR-BI deficient mice. Arterioscler Thromb Vasc Biol 27(4):858–864. https://doi.org/10.1161/01.ATV.0000259357.42089.dc
Hayek T, Chajek-Shaul T, Walsh A, Agellon LB, Moulin P, Tall AR, Breslow JL (1992) An interaction between the human cholesteryl ester transfer protein (CETP) and apolipoprotein A-I genes in transgenic mice results in a profound CETP-mediated depression of high density lipoprotein cholesterol levels. J Clin Invest 90(2):505–510. https://doi.org/10.1172/JCI115887
Hayek T, Masucci-Magoulas L, Jiang X, Walsh A, Rubin E, Breslow JL, Tall AR (1995) Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J Clin Invest 96(4):2071–2074. https://doi.org/10.1172/JCI118255
Inazu A, Brown ML, Hesler CB, Agellon LB, Koizumi J, Takata K, Maruhama Y, Mabuchi H, Tall AR (1990) Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med 323(18):1234–1238. https://doi.org/10.1056/NEJM199011013231803
Jiang XC, Beyer TP, Li ZQ, Liu J, Quan W, Schmidt RJ, Zhang YY, Bensch WR, Eacho PI, Cao GQ (2003) Enlargement of high density lipoprotein in mice via liver X receptor activation requires apolipoprotein E and is abolished by cholesteryl ester transfer protein expression. J Biol Chem 278(49):49072–49078. https://doi.org/10.1074/jbc.M304274200
Jiang XC, Bruce C, Mar J, Lin M, Ji Y, Francone OL, Tall AR (1999) Targeted mutation of plasma phospholipid transfer protein gene markedly reduced high-density lipoprotein levels. J Clin Invest 103(6):907–914. https://doi.org/10.1172/JCI5578
Jiang XC, Masucci-Magoulas L, Mar J, Lin M, Walsh A, Breslow JL, Tall A (1993) Down-regulation of mRNA for the low density lipoprotein receptor in transgenic mice containing the gene for human cholesteryl ester transfer protein Mechanism to explain accumulation of lipoprotein B particles. J Biol Chem 268(36):27406–12
Jiang XC, Zhou HW (2006) Plasma lipid transfer proteins. Curr Opin Lipidol 17(3):302–308. https://doi.org/10.1097/01.mol.0000226124.94757.ee
Kawano K, Qin SC, Lin M, Tall AR, Jiang XC (2000) Cholesteryl ester transfer protein and phospholipid transfer protein have nonoverlapping functions in vivo. J Biol Chem 275(38):29477–29481. https://doi.org/10.1074/jbc.M003523200
Lagrost L, Athias A, Gambert P, Lallemant C (1994) Comparative study of phospholipid transfer activities mediated by cholesteryl ester transfer protein and phospholipid transfer protein. J Lipid Res 35(5):825–835
Li J, Pijut SS, Wang Y, Ji A, Kaur R, Temel RE, van der Westhuyzen DR, Graf GA (2019) Simultaneous determination of biliary and intestinal cholesterol secretion reveals that CETP (cholesteryl ester transfer protein) alters elimination route in mice. Arterioscler Thromb Vasc Biol 39(10):1986–1995. https://doi.org/10.1161/ATVBAHA.119.312952
Li T, Yin JY, Ji YB, Lin P, Li YJ, Yang ZX, Hu SM, Wang J, Zhang BH, Koshti S, Wang JF, Ji CF, Guo SD (2020) Setosphapyrone C and D accelerate macrophages cholesterol efflux by promoting LXRα/ABCA1 pathway. Arch Pharm Res 43(8):788–797. https://doi.org/10.1007/s12272-020-01255-w
Low H, Hoang A, Sviridov D (2012) Cholesterol efflux assay. J Vis Exp 61:e3810. https://doi.org/10.3791/3810
Marotti KR, Castle CK, Murray RW, Rehberg EF, Polites HG, Melchior GW (1992) The role of cholesteryl ester transfer protein in primate apolipoprotein A-I metabolism. Insights from studies with transgenic mice. Arterioscler Thromb Vasc Biol 12(6):736–44. https://doi.org/10.1161/01.atv.12.6.736
Masson D, Staels B, Gautier T, Desrumaux C, Athias A, Le Guern N, Schneider M, Zak Z, Dumont L, Deckert V, Tall A, Jiang XC, Lagrost L (2004) Cholesteryl ester transfer protein modulates the effect of liver X receptor agonists on cholesterol transport and excretion in the mouse. J Lipid Res 45(3):543–550. https://doi.org/10.1194/jlr.M300432-JLR200
Masucci-Magoulas L, Plump A, Jiang XC, Walsh A, Breslow JL, Tall AR (1996) Profound induction of hepatic cholesteryl ester transfer protein transgene expression in apolipoprotein E and low density lipoprotein receptor gene knockout mice. A novel mechanism signals changes in plasma cholesterol levels. J Clin Invest 97(1):154–61. https://doi.org/10.1172/JCI118384
Oliveira HC, Ma L, Milne R, Marcovina SM, Inazu A, Mabuchi H, Tall AR (1997) Cholesteryl ester transfer protein activity enhances plasma cholesteryl ester formation. Studies in CETP transgenic mice and human genetic CETP deficiency. Arterioscler Thromb Vasc Biol. 17(6):1045–52. https://doi.org/10.1161/01.atv.17.6.1045
Ouimet M, Barrett TJ, Fisher EA (2019) HDL and reverse cholesterol transport. Circ Res 124(10):1505–1518. https://doi.org/10.1161/CIRCRESAHA.119.312617
Plump AS, Masucci-Magoulas L, Bruce C, Bisgaier CL, Breslow JL, Tall AR (1999) Increased atherosclerosis in apoE and LDL receptor gene knock-out mice as a result of human cholesteryl ester transfer protein transgene expression. Arterioscler Thromb Vasc Biol 19(4):1105–1110. https://doi.org/10.1161/01.atv.19.4.1105
Schwartz CC, Vanden Broek JM, Cooper PS (2004) Lipoprotein cholesteryl ester production, transfer, and output in vivo in humans. J Lipid Res 45(9):1594–1607. https://doi.org/10.1194/jlr.M300511-JLR200
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal-OUTCOMES Investigators, (2012) Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367(22):2089–2099. https://doi.org/10.1056/NEJMoa1206797 (Epub 2012 Nov 5)
Si YH, Zhang Y, Chen XF, Zhai L, Zhou GH, Yu AL, Cao HJ, Shucun Q (2016) Phospholipid transfer protein deficiency in mice impairs macrophage reverse cholesterol transport in vivo. Exp Biol Med (Maywood) 241(13):1466–1472. https://doi.org/10.1177/1535370216641218
Siggins S, Bykov I, Hermansson M, Somerharju P, Lindros K, Miettinen TA, Jauhiainen M, Olkkonen VM, Ehnholm C (2007) Altered hepatic lipid status and apolipoprotein A-I metabolism in mice lacking phospholipid transfer protein. Atherosclerosis 190(1):114–123. https://doi.org/10.1016/j.atherosclerosis.2006.02.037
Tanigawa H, Billheimer JT, Tohyama J, Fuki IV, Ng DS, Rothblat GH, Rader DJ (2009) Lecithin: cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation 120(2):160–169. https://doi.org/10.1161/CIRCULATIONAHA.108.825109
Tanigawa H, Billheimer JT, Tohyama J, Zhang YZ, Rothblat G, Rader DJ (2007) Expression of cholesterol ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation 116(11):1267–1273. https://doi.org/10.1161/CIRCULATIONAHA.107.704254
Thompson A, Di Angelantonio E, Sarwar N, Erqou S, Saleheen D, Dullaart RP, Keavney B, Ye Z, Danesh J (2008) Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA 299(23):2777–2788. https://doi.org/10.1001/jama.299.23.2777
Tian H, Liu QC, Qin SC, Zong CL, Zhang Y, Yao ST, Yang NN, Guan T, Guo SD (2017) Synthesis and cardiovascular protective effects of quercetin 7-O-sialic acid. J Cell Mol Med 21(1):107–120. https://doi.org/10.1111/jcmm.12943
Van Capelleveen JC, Kastelein JJ, Zwinderman AH, van Deventer SJ, Collins HL, Adelman SJ, Round P, Ford J, Rader DJ, Hovingh GK (2016) Effects of the cholesteryl ester transfer protein inhibitor, TA-8995, on cholesterol efflux capacity and high-density lipoprotein particle subclasses. J clin Lipidol 10(5):1137-1144.e3. https://doi.org/10.1016/j.jacl.2016.06.006
Westerterp M, van der Hoogt CC, de Haan W, Offerman EH, Dallinga-Thie GM, Jukema JW, Havekes LM, Rensen PC (2006) Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in apoE*3-leiden mice. Arterioscler Thromb Vasc Biol 26(11):2552–2559. https://doi.org/10.1161/01.ATV.0000243925.65265.3c
Yang ZX, Liu GJ, Wang YF, Yin JY, Wang J, Xia B, Li T, Yang XQ, Hou PB, Hu SM, Song WG, Guo SD (2019) Fucoidan A2 from the brown seaweed Ascophyllum nodosum lowers lipid by improving reverse cholesterol transport in C57BL/6J mice fed a high-fat diet. J Agric Food Chem 67:5782–5791. https://doi.org/10.1021/acs.jafc.9b01321
Yao ST, Tian H, Zhao L, Li JG, Yang LB, Yue F, Li YY, Jiao P, Yang NN, Wang YW, Zhang XJ, Qin SC (2017) Oxidized high density lipoprotein induces macrophage apoptosis via toll-like receptor 4-dependent CHOP pathway. J Lipd Res 58(1):164–177. https://doi.org/10.1194/jlr.M071142
Zhang M, Charles R, Tong HM, Zhang L, Patel M, Wang F, Rames MJ, Ren A, Rye KA, Qiu XY, Johns DG, Charles MA, Ren G (2015) HDL surface lipids mediate CETP binding as revealed by electron microscopy and molecular dynamics simulation. Sci Rep 5:8741. https://doi.org/10.1038/srep08741
Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR (1996) Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest 97(12):2917–2923. https://doi.org/10.1172/JCI118751
Acknowledgements
We thank Xian-cheng Jiang at SUNY Downstate Medical Center (USA) for providing PLTP knockout and CETP transgenic mice. We also thank Guang-hai Zhou at Shandong First Medical University & Shandong Academy of Medical Sciences for providing help in detection of [3H]-cholesterol.
Funding
This work was supported by Natural Science Foundation of China (81770463, 82070469 and 81600681) and Taishan Scholars Foundation of Shandong Province (ts201511057).
Author information
Authors and Affiliations
Contributions
Na Liu performed the Western Blotting experiment; Yanhong Si and Ying Zhang carried out the animal treatment, data acquirement, and analysis; Shoudong Guo performed the LC–MS/MS analysis, funding, and writing of this manuscript; Shucun Qin provided funding and animal for this study. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Informed consent
This study was approved by the laboratory animals’ ethical committee of Taishan Medical University and followed national guidelines for the care and use of animals.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• CETP transgene improves macropahge reverse cholesterol transport in C57/BL/6 J mice.
• CETP improves macropahge reverse cholesterol transport in PLTP knockout mice.
• CETP transgene has no effect on RCT-related proteins expression.
• CETP transgene changes the lipid profiles of the mice plasma and lipoproteins.
Rights and permissions
About this article
Cite this article
Liu, N., Si, Y., Zhang, Y. et al. Human cholesteryl ester transport protein transgene promotes macrophage reverse cholesterol transport in C57BL/6 mice and phospholipid transfer protein gene knockout mice. J Physiol Biochem 77, 683–694 (2021). https://doi.org/10.1007/s13105-021-00834-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00834-9