Abstract
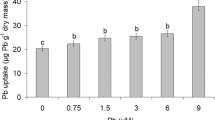
Bicarbonate (HCO3−) is one of the dominant carbon forms in the aquatic ecosystem and carbon cycle in karst areas. Recent studies have focused on the HCO3− utilization by aquatic plants to assess karst carbon sink at a watershed scale. However, the predominated researches inadequately address the effects on growth of submerged plants in various HCO3− conditions and how the submerged plants affect the karst carbon cycle. Here, H. verticillata was selected as a research object in different HCO3− concentrations. Plant morphology and water chemistry were analyzed to comparatively study the growth strategy and carbon utilization of submerged plants under various HCO3− conditions. The results show that the HCO3− aquatic environment can remarkably promote the growth of H. verticillata in terms of biomass, apical shoots, lateral shoots, and root production. The optimum concentration on biomass accumulation and lateral shoots growth is 4 mmol L−1; while, it is 8 mmol L−1 for the apical growth. But overall, the biomass accumulation is one order of magnitude higher than that in the control group. Despite the abundant dissolved inorganic carbon can significantly stimulate the growth of submerged plants, the respiration of H. verticillata suffers a certain inhabitation when the HCO3− concentration exceeds 4 mmol L−1. In karst aquatic environment, the existing HCO3− not only promotes the growth of submerged plants by means of supplying abundant dissolved inorganic carbon but also creates an alkaline water environment to buffer CO2 from the atmosphere. As a consequence, the presence of submerged plants has greatly enhanced the stability of karst carbon sink.






Similar content being viewed by others
References
Amthor JS, Koch GW, Bloom AJ (1992) CO2 inhibits respiration in leaves of Rumex crispus L. Plant Physiol 98(2):757–760
Barko JW, Adams MS, Clesceri NL (1986) Environmental factors and their consideration in the management of submersed aquatic vegetation: a review. J Aquat Plant Manag 24:1–10
Berner RA, Lasaga AC, Garrells RM (1983) The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am J Sci 283:641–683
Bowes G (1991) Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant Cell Environ 14:795–806
Bowes G (2011) Single-cell C4 photosynthesis in aquatic plants. Chapter 5. In: Raghavendra AS, Sage RF (eds) C4 photosynthesis and related CO2 concentrating mechanisms. Springer, Berlin, pp 63–80
Bowes G, Holaday AS, Haller WT (1979) Seasonal variation in the biomass, tuber density, and photosynthetic metabolism of Hydrilla in three Florida lakes. J Aquat Plant Manag 17:61–65
Cao JH, Yang H, Kang ZQ (2011) Preliminary regional estimation of carbon sink flux by carbonate rock corrosion: a case study of the Pearl River Basin. Chin Sci Bull 56(35):3766–3773
Cao JH, Yuan DX, Chris G, Huang F, Yang H (2012) Carbon fluxes and sinks: the consumption of atmospheric and soil CO2 by carbonate rock dissolution. Acta Geol Sin 86(4):963–972
Cao JH, Hu B, Groves C, Huang F, Yang H, Zhang CL (2016) Karst dynamic system and the carbon cycle. Z Geomorphol 60(2):035–055
Carpenter SR, Lodge DM (1986) Effects of submersed macrophytes on ecosystem processes. Aquat Bot 26:341–370
Cole JJ, Prairie YT, Caraco NF, Mcdowell WH, Tranvik LJ, Striegl RG, Duarte CM, Kortelainen P, Dowing JA, Middelburg JJ, Melack J (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10(1):171–184
Curl RL (2012) Carbon shifted but not sequestered. Science 335(6069):655–655
de Montety V, Martin JB, Cohen MJ, Foster C, Kurz MJ (2011) Influence of diel biogeochemical cycles on carbonate equilibrium in a karst river. Chem Geol 283(1):31–43
Dean WE, Gorham E (1998) Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 26(6):535–538
Dou Y, Wang B, Chen L, Yin D (2013) Alleviating versus stimulating effects of bicarbonate on the growth of Vallisneria natans under ammonia stress. Environ Sci Pollut Res 20(8):5281–5288
Falkowski P, Scholes RJ, Boyle E (2000) The global carbon cycle: a test of our knowledge of earth as a system. Science 290(5490):291–296
Gillard M, Thiébaut G, Rossignol N, Berardocco S, Deleu C (2017) Impact of climate warming on carbon metabolism and on morphology of invasive and native aquatic plant species varies between spring and summer. Environ Exp Bot 144:1–10
Gombert P (2002) Role of karstic dissolution in global carbon cycle. Glob Planet Change 33(1–2):177–184
Gonzàlez-Meler MA, Blanc-Betes E, Flower CE, Ward JK, Gomez-Casanovas N (2009) Plastic and adaptive responses of plant respiration to changes in atmospheric CO2 concentration. Physiol Plant 137(4):473–484
Holaday AS, Bowes G (1980) C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiol 65(2):331–335
Huang F, Zhang CL, Xie YC, Li L, Cao JH (2015) Inorganic carbon flux and its source in the catchment of Maocun, Guilin, China. Environ Earth Sci 74(2):1079–1089
Hunt R (2003) Growth and development|growth analysis, individual plants. Encycl Appl Plant Sci 152(3862):579–588
Hunt R (2012) Basic growth analysis: plant growth analysis for beginners. Springer Science & Business Media, London
Jeppesen E, Sondergaard M, Sondergaard M, Christofferson K (eds) (2012) The structuring role of submerged macrophytes in lakes, vol 131. Springer Science & Business Media, London
Jiang Y, Hu Y, Schirmer M (2013) Biogeochemical controls on daily cycling of hydrochemistry and δ13C of dissolved inorganic carbon in a karst spring-fed pool. J Hydrol 478:157–168
Jones JI, Eaton JW, Hardwick K (1993) Physiological plasticity in Elodea nuttallii (Planch.) St. John. J Aquat Plant Manag 31:88–94
Jones JI, Young JO, Eaton JW, Moss B (2002) The influence of nutrient loading, dissolved inorganic carbon and higher trophic levels on the interaction between submerged plants and periphyton. J Ecol 90(1):12–24
Larson C (2011) An unsung carbon sink. Science 334:886–887
Li SL, Liu CQ, Lang YC, Tao EX, Zhao ZQ, Zhou ZH (2008) Stable carbon isotope biogeochemistry and anthropogenic impacts on karst ground water, Zunyi, Southwest China. Aquat Geochem 14(3):211–221
Liu ZH, Dreybrodt W, Wang HJ (2010) A new direction in effective accounting for the atmospheric CO2 budget: considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC by aquatic organisms. Earth Sci Rev 99(3):162–172
Longstreth DJ (1989) Photosynthesis and photorespiration in freshwater emergent and floating plants. Aquat Bot 34(1–3):287–299
Maberly SC, Madsen TV (2002a) Use of bicarbonate ions as a source of carbon in photosynthesis by Callitriche hermaphroditica. Aquat Bot 73(1):1–7
Maberly SC, Madsen TV (2002b) Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Funct Plant Biol 29(3):393–405
Maberly SC, Spence DHN (1983) Photosynthetic inorganic carbon use by freshwater plants. J Ecol 73:705–724
Maberly SC, Spence DHN (1989) Photosynthesis and photorespiration in freshwater organisms: amphibious plants. Aquat Bot 34(1–3):267–286
Maberly SC, Berthelot SA, Stott AW, Gontero B (2015) Adaptation by macrophytes to inorganic carbon down a river with naturally variable concentrations of CO2. J Plant Physiol 172:120–127
Madsen T, Sand-Jensen K (1994) Photosynthetic carbon assimilation in aquatic macrophytes. Aquat Bot 41:5–40
Magnin NC, Cooley BA, Reiskind JB, Bowes G (1997) Regulation and localization of key enzymes during the induction of Kranz-less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiol 115(4):1681–1689
Moore BD, Ku MSB, Edwards GE (1987) C4 photosynthesis and light dependent accumulation of inorganic carbon in leaves of C3–C4 Flaveria species. Aust J Plant Physiol 14(6):657–688
Mulsow S, Grandjean M (2006) Incompatibility of sulphate compounds and soluble bicarbonate salts in the Rio Cruces water: an answer to the disappearance of Egeria densa and black-necked swans in RAMSAR sanctuary. Ethics Sci Environ Politics 6(1):5–11
Nimick DA, Gammons CH, Cleasby TE, Madison JP, Skaar D, Brick CM (2003) Diel cycles in dissolved metal concentrations in streams: occurrence and possible causes. Water Resour Res 39:333–342
Pedersen O, Colmer TD, Sand-Jensen K (2013) Underwater photosynthesis of submerged plants-recent advances and methods. Front Plant Sci 4:1–19
Pu JB, Li JH, Khadka MB, Martin JB, Zhang T, Yu S, Yuan DX (2017) In-stream metabolism and atmospheric carbon sequestration in a groundwater-fed karst stream. Sci Total Environ 579:1343–1355
Rosa M, Prado C, Podazza G, Interdonato González JA, Hilal M, Prado FE (2009) Soluble sugars: metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signal Behav 4(5):388–393
Spence DHN, Maberly SC (1985) Occurrence and ecological importance of HCO3− use among aquatic higher plants. In: Lucas WJ, Berry JA, eds. Inorganic carbon uptake by aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 125–143
Strik WA, Kulkarni MG, Staden J (2016) Effect of smoke-derived extracts on Spirodela polyrhiza, an aquatic plant grown in nutrient-rich and -depleted conditions. Aquat Bot 129:31–34
Stumm W, Morgan JJ (2012) Aquatic chemistry, 3rd edn. Wiley, New York
Wang P, Hu QJ, Yang H, Cao JH, Li L, Liang Y, Wang KR (2014) Preliminary study on the utilization of Ca2+ and HCO3− in karst water by different sources of Chlorella vulgaris. Carbonate Evaporite 29(2):203–210
Wang SL, Yeager KM, Wan GJ, Liu CQ, Liu F, Lü YC (2015) Dynamics of CO2 in a karst catchment in the southwestern plateau, China. Environ Earth Sci 73(5):2415–2427
Wang P, Cao JH, Shao JL (2017a) Effects of typical aquatic plants on the stability of inorganic carbon in karst aquatic ecosystem. Acta Geosci Sin 38:51–54 (in Chinese with English abstract)
Wang P, Hu G, Cao JH (2017b) Stable carbon isotopic composition of submerged plants living in karst water and its eco-environmental importance. Aquat Bot 140:78–83
Wang WF, Li SL, Zhong J, Maberly SC, Li C, Wang FS, Xiao HY, Liu CQ (2019) Climatic and anthropogenic regulation of carbon transport and transformation in a karst river-reservoir system. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.135628
Yuan DX (1993) Carbon cycle and global karst. Quat Sci 1:1–6
Yuan DX (1997a) The carbon cycle in karst. Z Geomorphol 108:91–102
Yuan DX (1997b) Modern karstology and global change study. Earth Sci Front 4:17–25 (in Chinese with English abstract)
Zavadlav S, Kanduč T, McIntosh J, Lojen S (2013) Isotopic and chemical constraints on the biogeochemistry of dissolved inorganic carbon and chemical weathering in the Karst watershed of Krka River (Slovenia). Aquat Geochem 19:209–230
Zhang C, Wang JL, Pu JB (2015) Diel aqueous chemical cycling in a typical karst spring-fed stream: controls of biogeochemical process. Acta Geosci Sin 36(2):197–203
Acknowledgements
We would like to thank Li Guangchao for his assistance with English proof-reading of the manuscript. This study was supported by the National Natural Science Foundation of China (41807205), the National Key Research and Development Plan of China (2016YFC0502506 and 2016YFC0500403-03), the Chinese Geological Survey Project (12120113005300), Guangxi Science and Technology Development Program (1598023-1) and the Karst Dynamics Laboratory, MLR and GZAR (KDL201304).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, P., Zhang, X., Wang, D. et al. Experimental study on growth of Hydrilla verticillata under different concentrations of bicarbonate and its implication in karst aquatic ecosystem. Carbonates Evaporites 35, 83 (2020). https://doi.org/10.1007/s13146-020-00618-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s13146-020-00618-0