Abstract
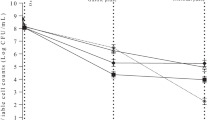
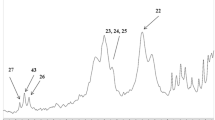
Nowadays, the strong relationship between diet and health is well known. Although the primary role of diet is to provide nutrients to fulfill metabolic requirements, the use of foods to improve health and the state of well-being is an idea increasingly accepted by society in the last three decades. During the last years, an important number of scientific advances have been achieved in this field and, although in some situations, it is difficult to establish a distinction between “harmful” and “good” bacteria, experts agree in classifying the genera Bifidobacterium and Lactobacillus as beneficial bacteria. Thus, several strategies can be used to stimulate the proliferation of these beneficial intestinal bacteria, being one of them the consumption of prebiotics. The development of new prebiotics, with added functionality, is one of the most serious challenges shared not only by the scientific community but also by the food industry. The objective of this work was to evaluate the potential prebiotic effect of red and white grape residues, both obtained during the winemaking process. For such purpose, an in vitro study with pure cultures of Lactobacillus and Bifidobacterium was first conducted. Secondly, a study with mixed cultures using human fecal inocula was carried out in a simulator of the distal part of the colon. The obtained results showed an increase in the Lactobacillus and Bifidobacterium population, indicating that these ingredients are serious candidates to be considered as prebiotics.


Similar content being viewed by others
References
Barroso E, Cueva C, Peláez C, Martinez-Cuesta MC, Requena T (2015) Development of human colonic microbiota in the computer-controlled dynamic simulator of the GastroIntestinal tract SIMGI. LWT-Food Sci Tech 61:283–289
Barroso E, Sanchez-Patan F, Martin-Alvarez PJ, Bartolome B, Moreno-Arribas MV et al (2013) Lactobacillus plantarum IFPL935 favors the initial metabolism of red wine polyphenols when added to a colonic microbiota. J Agr Food Chem 61:10163–10172
Cardelle-Cobas A, Olano A, Corzo N, Villamiel M, Collins M et al (2012) In vitro fermentation of lactulose-derived oligosaccharides by mixed fecal microbiota. J Agr Food Chem 30:2024–2032
Cueva C, Sánchez-Patán F, Monagas M, Walton GE, Gibson GR et al (2012) In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: changes in microbial groups and phenolic metabolites. FEMS Microbiol Ecol 83:792–805
Dávalos A, Asunción MA (2009) Health-promoting effects of wine phenolics. En “Wine chemistry and biochemistry” (Moreno-Arribas, M. V., Polo, M. C. Eds.), Springer, New York, pp. 571–591
Demrow HS, Slane PR, Folts JD (1995) Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation 91:1182–1188
Dolara P, Luceri C, De Filippo C, Femia AP, Giovannelli L et al (2005) Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonic mucosa in F344 rats. Mut Res - Fund Molec Mech Mutag 591:237–246
Dueñas M, Cueva C, Muñoz-González I, Jimenez-Girón A, Sánchez-Patán F et al (2015) Studies on modulation of gut microbiota by wine polyphenols: from isolated cultures to omic approaches. Antioxidants 4:1–2
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120
Friswell MK, Gika H, Stratford IJ, Theodoridis G, Telfer B, Wilson ID, McBain AJ (2010) Site and strain-specific variation in gut microbiota profiles and metabolism in experimental mice. PLoS One 5(1):e8584 1-10
Gullón B, Gomez B, Martínez-Sabajanes M, Yañez R, Parajó JC et al (2013) Pectic oligosaccharides: manufacture and functional properties. Trends Food Sci Technol 30:153–161
Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K (2008) Development of a realtime PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 47:367–373
Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD et al (2002) Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol 68:114–123
Hermann-Bank ML, Skovgaard K, Stockmarr A, Larsen N, Molbak L (2013) The gut microbiotassay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. Bio Med Central Genetics 14:788–802
Kemperman RA, Gross G, Mondot S, Possemiers S, Marzorati M et al (2013) Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res Int 53:659–669
Korhonen H (2002) Technology options for new nutritional concepts. Int J Dair Technol 55:79–88
Layton A, MacKay L, Willians D, Garret V, Gentry R et al (2006) Development of Bacteroides 16S rRNA gene TaqMan-based real-time PCR assays for estimation of total, human and bovine fecal pollution in water. Appl Environ Microbiol 72:4214–4224
Lee HC, Jenner AM, Seng Low C, Kun Lee Y (2006) Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol 157:876–884
Lee ZMP, Bussema C, Schmidt TM (2009) rrnDB: documenting the number of rRNA and tRNA genes in bacteria and archaea. Nucl Ac Res 37:489–493
Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R (2004) Use of 16S rRNA genetargeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol 70:7220–7228
Minekus M, Alminger M, Alvito P, Balance S, Bohn T et al (2014) A standardised static in vitro digestion method suitable for food-an international consensus. Food Func 5:1113–1124
Monagas M, Bartolomé B, Gómez-Cordovés C (2005) Updated knowledge about the presence of phenolic compounds in wine. Crit Rev Food Sci Nutr 45:85–118
Monagas M, Urpi-Sarda M, Sánchez-Patán F, Llorach R, Garrido I et al (2010) Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct 1:233–253
Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F et al (2013) Gut microbiota in children with type I diabetes differs from that in healthy children: a case-control study. Bio Med Central Medicine 11:46–57
Rastall RA, Maitin V (2002) Prebiotics and synbiotics: towards the next generation. Curr Opin Biotechnol 13:490–496
Ruiz-Matute AI, Brokl M, Sanz ML, Soria AC, Côté GL et al (2011) Effect of dextransucrase cellobiose acceptor products on the growth of human gut bacteria. J Agr Food Chem 59:3693–3700
Russo P, Tedesco I, Russo M, Russo GL, Venezia A et al (2001) Effects of de-alcoholated red wine and its phenolic fractions on platelet aggregation. Nutr Metabol Card Dis 11:25–29
Saito M, Hosoyama H, Ariga T, Kataoka S, Yamaji N (1998) Antiulcer activity of grape seed extract and procyanidins. J Agr Food Chem 46:1460–1464
Sánchez-Patán F, Barroso E, van de Wiele T, Jiménez-Girón A, Martín-Álvarez PJ et al (2015) Comparative in vitro fermentations of cranberry and grape seed polyphenols with colonic microbiota. Food Chem 183:273–282
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306
Tabasco R, Sánchez-Patán F, Monagas M, Bartolomé B, Moreno-Arribas MV et al (2011) Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: resistance and metabolism. Food Microbiol 28:1345–1352
Villanueva-Millán MJ, Pérez-Matute P, Oteo JA (2015) Gut microbiota: a key player in health and disease. A review focused in obesity. J Physiol Biochem 71:509–525
Xu Y (2001) Perspectives on the 21st century development of functional foods: bridging Chinese medicated diet and functional foods. Int J Food Sci Technol 36:229–242
Yamakoshi J, Tokutake S, Kikuchi M, Kubota Y, Konishi H et al (2001) Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora. Micr Ecol Health Dis 13:25–31
Acknowledgements
Cardelle-Cobas thanks the Spanish Ministry of Economy and Competitivity for her postdoctoral contract with reference FPDI-2013-18601.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no conflicts of interests.
Additional information
This article forms part of a special issue of the Journal of Physiology and Biochemistry entitled “Impact of lifestyles patterns on human health: Integrated approach from the child to the elderly”
Rights and permissions
About this article
Cite this article
Rodríguez-Costa, S., Cardelle-Cobas, A., Roca-Saavedra, P. et al. In vitro evaluation of the prebiotic effect of red and white grape polyphenolic extracts. J Physiol Biochem 74, 101–110 (2018). https://doi.org/10.1007/s13105-017-0573-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-017-0573-1