Abstract
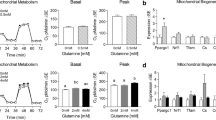
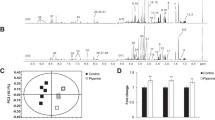
Caffeine has been shown to stimulate multiple major regulators of cell energetics including AMP-activated protein kinase (AMPK) and Ca2+/calmodulin-dependent protein kinase II (CaMKII). Additionally, caffeine induces peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and mitochondrial biogenesis. While caffeine enhances oxidative metabolism, experimental concentrations often exceed physiologically attainable concentrations through diet. This work measured the effects of low-level caffeine on cellular metabolism and gene expression in myotubes, as well as the dependence of caffeine’s effects on the nuclear receptor peroxisome proliferator-activated receptor beta/delta (PPARβ/δ). C2C12 myotubes were treated with various doses of caffeine for up to 24 h. Gene and protein expression were measured via qRT-PCR and Western blot, respectively. Cellular metabolism was determined via oxygen consumption and extracellular acidification rate. Caffeine significantly induced regulators of mitochondrial biogenesis and oxidative metabolism. Mitochondrial staining was suppressed in PPARβ/δ-inhibited cells which was rescued by concurrent caffeine treatment. Caffeine-treated cells also displayed elevated peak oxidative metabolism which was partially abolished following PPARβ/δ inhibition. Similar to past observations, glucose uptake and GLUT4 content were elevated in caffeine-treated cells, however, glycolytic metabolism was unaltered following caffeine treatment. Physiological levels of caffeine appear to enhance cell metabolism through mechanisms partially dependent on PPARβ/δ.






Similar content being viewed by others
Abbreviations
- ACAD:
-
Fatty acyl-coA dehydrogenase
- ACC:
-
Acetyl-coA carboxylase
- AMPK:
-
AMP-activated protein kinase
- CaMKI/II:
-
Ca2+/calmodulin-dependent protein kinases I/II
- ChREBP:
-
Carbohydrate-responsive element-binding protein
- CREB:
-
cAMP response element-binding protein
- CS:
-
Citrate synthase
- ERRα:
-
Estrogen-related receptor alpha
- FAS:
-
Fatty acid synthase
- Foxo1:
-
Forkhead box protein O1
- GLUT4:
-
Glucose transporter 4
- LDHa:
-
Lactate dehydrogenase a
- LDHb:
-
Lactate dehydrogenase b
- MEF2:
-
Myocyte enhancer factor 2
- NRF1:
-
Nuclear respiratory factor 1
- PGC-1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPARα:
-
Peroxisome proliferator-activated receptor alpha
- PPARβ/δ:
-
Peroxisome proliferator-activated receptor beta/delta
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- SCD1:
-
Stearoyl-CoA desaturase
- SREBP-1c:
-
Sterol receptor element-binding protein-1c
- SIRT3:
-
NAD+-dependent deacetylase sirtuin 3
- TFAM:
-
Mitochondrial transcription factor A
- UCP:
-
Uncoupling protein
References
Abbott MJ, Bogachus LD, Turcotte LP (2011) AMPKalpha2 deficiency uncovers time dependency in the regulation of contraction-induced palmitate and glucose uptake in mouse muscle. J Appl Physiol 111(1):125–134. https://doi.org/10.1152/japplphysiol.00807.2010
Abbott MJ, Edelman AM, Turcotte LP (2009) CaMKK is an upstream signal of AMP-activated protein kinase in regulation of substrate metabolism in contracting skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297(6):R1724–R1732. https://doi.org/10.1152/ajpregu.00179.2009
Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ, Zierath JR (2012) Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15(3):405–411. https://doi.org/10.1016/j.cmet.2012.01.001
Brenmoehl J, Hoeflich A (2013) Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 13(6):755–761. https://doi.org/10.1016/j.mito.2013.04.002
Egawa T, Hamada T, Kameda N, Karaike K, Ma X, Masuda S, Iwanaka N, Hayashi T (2009) Caffeine acutely activates 5 ′ adenosine monophosphate-activated protein kinase and increases insulin-independent glucose transport in rat skeletal muscles. Metab Clin Exp 58(11):1609–1617. https://doi.org/10.1016/j.metabol.2009.05.013
Egawa T, Hamada T, Ma X, Karaike K, Kameda N, Masuda S, Iwanaka N, Hayashi T (2011) Caffeine activates preferentially alpha 1-isoform of 5 ′ AMP-activated protein kinase in rat skeletal muscle. Acta Physiol 201(2):227–238. https://doi.org/10.1111/j.1748-1716.2010.02169.x
Evans MJ, Scarpulla RC (1990) NRF-1—a transactivator of nuclear-encoded respiratory genes in animal-cells. Genes Dev 4(6):1023–1034. https://doi.org/10.1101/gad.4.6.1023
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25(4):1354–1366. https://doi.org/10.1128/MCB.25.4.1354-1366.2005
Graham TE (2001) Caffeine and exercise: metabolism, endurance and performance. Sports Med 31(11):785–807. https://doi.org/10.2165/00007256-200131110-00002
Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM (2008) Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol 49(5):758–767. https://doi.org/10.1016/j.jhep.2008.03.029
Hashimoto T, Sato K, Iemitsu M (2013) Exercise-inducible factors to activate lipolysis in adipocytes. J Appl Physiol 115(2):260–267. https://doi.org/10.1152/japplphysiol.00427.2013
Kim AR, Yoon BK, Park H, Seok JW, Choi H, JH Y, Choi Y, Song SJ, Kim A, Kim JW (2016) Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep 49(2):111–115. https://doi.org/10.5483/BMBRep.2016.49.2.128
Lally JS, Jain SS, Han XX, Snook LA, Glatz JF, Luiken JJ, McFarlan J, Holloway GP, Bonen A (2012) Caffeine-stimulated fatty acid oxidation is blunted in CD36 null mice. Acta Physiol 205(1):71–81. https://doi.org/10.1111/j.1748-1716.2011.02396.x
McConell GK, Ng GPY, Phillips M, Ruan Z, Macaulay SL, Wadley GD (2010) Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J Appl Physiol 108(3):589–595. https://doi.org/10.1152/japplphysiol.00377.2009
Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM (2001) Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci 98(7):3820–3825. https://doi.org/10.1073/pnas.061035098
Miyake T, McDermott JC, Gramolini AO (2011) A method for the direct identification of differentiating muscle cells by a fluorescent mitochondrial dye. PLoS One 6(12):1–9
Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA (2008) CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J Biol Chem 283(21):14317–14326. https://doi.org/10.1074/jbc.M706478200
Neels JG, Grimaldi PA (2014) Physiological functions of peroxisome proliferator-activated receptor beta. Physiol Rev 94(3):795–858. https://doi.org/10.1152/physrev.00027.2013
Ojuka EO (2004) Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc 63(2):275–278. https://doi.org/10.1079/PNS2004339
Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO (2003) Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J 17(6):675–681. https://doi.org/10.1096/fj.02-0951com
Ojuka EO, Jones TE, Han DH, Chen M, Wamhoff BR, Sturek M, Holloszy JO (2002) Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol Endocrinol Metab 283(5):E1040–E1045. https://doi.org/10.1152/ajpendo.00242.2002
Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO (2002) Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca(2+). Am J Physiol Endocrinol Metab 282(5):E1008–E1013. https://doi.org/10.1152/ajpendo.00512.2001
Palkar PS, Borland MG, Naruhn S, Ferry CH, Lee C, Sk UH, Sharma AK, Amin S, Murray IA, Anderson CR, Perdew GH, Gonzalez FJ, Muller R, Peters JM (2010) Cellular and pharmacological selectivity of the peroxisome proliferator-activated receptor-beta/delta antagonist GSK3787. Mol Pharmacol 78(3):419–430. https://doi.org/10.1124/mol.110.065508
Quan HY, Kim DY, Chung SH (2013) Caffeine attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. BMB Rep 46(4):207–212. https://doi.org/10.5483/BMBRep.2013.46.4.153
Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M (2010) A review of the herbal phosphodiesterase inhibitors: future perspective of new drugs. Cytokine 49(2):123–129. https://doi.org/10.1016/j.cyto.2009.11.005
Raney MA, Turcotte LP (2008) Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J Appl Physiol 104(5):1366–1373. https://doi.org/10.1152/japplphysiol.01282.2007
Scarpulla RC (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta 1813(7):1269–1278. https://doi.org/10.1016/j.bbamcr.2010.09.019
Scarpulla RC (2002) Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene 286(1):81–89. https://doi.org/10.1016/S0378-1119(01)00809-5
Schnuck JK, Sunderland KL, Gannon NP, Kuennen MR, Vaughan RA (2016) Leucine stimulates PPARbeta/delta-dependent mitochondrial biogenesis and oxidative metabolism with enhanced GLUT4 content and glucose uptake in myotubes. Biochimie 128(1):1–7
Su SH, Shyu HW, Yeh YT, Chen KM, Yeh H, Su SJ (2013) Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol in Vitro 27(6):1830–1837. https://doi.org/10.1016/j.tiv.2013.05.011
Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA, Conn CA (2012) Effects of caffeine on metabolism and mitochondria biogenesis in rhabdomyosarcoma cells compared with 2,4-dinitrophenol. Nutr Metab Insights 13(5):59–70
Vega RB, Huss JM, Kelly DP (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Bio 20(5):1868–1876. https://doi.org/10.1128/MCB.20.5.1868-1876.2000
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors—a potential regulatory link between nuclear and mitochondrial gene-expression and organelle biogenesis. Proc Natl Acad Sci 91(4):1309–1313. https://doi.org/10.1073/pnas.91.4.1309
Wang YX, Zhang CL, RT Y, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM (2004) Regulation of muscle fiber type and running endurance by PPAR delta. PLoS Biol 2(10):1532–1539
Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO (2007) Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1 alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282(26):18793–18799. https://doi.org/10.1074/jbc.M611252200
Acknowledgments
We would like to thank the Department of Physical Therapy (Congdon School of Health Sciences) and the School of Arts and Sciences for the use of shared lab space and equipment.
Funding
Support for this work was provided by the Department of Exercise Science within the Congdon School of Health Sciences.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. JKS, LMG, HAP, MAJ, and NPG assisted with experiments and manuscript preparation. KLS assisted with experimental design and manuscript preparation. RAV performed and oversaw all experiments, statistical analyses, manuscript preparation, and designed/conceived the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Figure S1
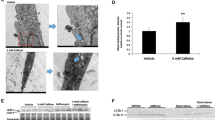
Cell viability. WST-1 assay time trial of cells treated with or without caffeine at 100 μM for 24 hours (left) and corresponding area-under-the-curve (right). Notes: Viability was performed using 10 replicates per treatment and analyzed using two-way ANOVA with Bonferroni’s correction for multiple comparisons. (JPEG 40 kb)
Rights and permissions
About this article
Cite this article
Schnuck, J.K., Gould, L.M., Parry, H.A. et al. Metabolic effects of physiological levels of caffeine in myotubes. J Physiol Biochem 74, 35–45 (2018). https://doi.org/10.1007/s13105-017-0601-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-017-0601-1