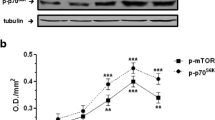
Abstract
Muscle cells adjust their glucose metabolism in response to myriad stimuli, and particular attention has been paid to glucose metabolism after contraction, ATP depletion, and insulin stimulation. Each of these requires translocation of GLUT4 to the cell membrane, and may require activation of glucose transporters by p38. In contrast, AICAR stimulates glucose transport without activation of p38, suggesting that p38 activation may be an indirect consequence of accelerated glucose transport or metabolism. This study was designed to investigate the contribution of AMPK and p38 to ATP homeostasis and glucose metabolism to test the hypothesis that p38 reflects glycolytic activity rather than controls glucose uptake. Treating mature myotubes with rotenone caused transient ATP depletion in 15 min with recovery by 120 min, associated with increased lactate production. Both ACC and p38 were rapidly phosphorylated, but ACC remained phosphorylated while p38 phosphorylation declined as ATP recovered. AMPK inhibition blocked ATP recovery, lactate production, and phosphorylation of p38 and ACC. Inhibition of p38 had little effect. AICAR induced ACC phosphorylation, but not lactate production or p38 phosphorylation. Finally, removing extracellular glucose potentiated rotenone-induced AMPK activation, but reduced lactate generation, ATP recovery and p38 activation. Thus, glucose metabolism is highly sensitive to ATP homeostasis via AMPK activity, but p38 activity is dispensable. Although p38 is strongly phosphorylated during ATP depletion, this appears to be an indirect consequence of accelerated glycolysis.








Similar content being viewed by others
References
Antonescu CN, Huang C, Niu W, Liu Z, Eyers PA, Heidenreich KA, Bilan PJ, Klip A (2005) Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 is independent of p38MAPK activity. Endocrinology 146:3773–3781
Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P (2007) The selectivity of protein kinase inhibitors: a further update. Biochem J 408:297–315
Bashan N, Burdett E, Guma A, Sargeant R, Tumiati L, Liu Z, Klip A (1993) Mechanisms of adaptation of glucose transporters to changes in the oxidative chain of muscle and fat cells. Am J Physiol 264:C430–C440
Becatti M, Taddei N, Cecchi C, Nassi N, Nassi PA, Fiorillo C (2012) SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol Life Sci 69:2245–2260
Boergermann JH, Kopf J, Yu PB, Knaus P (2010) Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol 42:1802–1807
Dehne N, Kerkweg U, Otto T, Fandrey J (2007) The HIF-1 response to simulated ischemia in mouse skeletal muscle cells neither enhances glycolysis nor prevents myotube cell death. Am J Physiol Regul Integr Comp Physiol 293:R1693–R1701
Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H (2003) A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A 100:11753–11758
Fendt SM, Bell EL, Keibler MA, Olenchock BA, Mayers JR, Wasylenko TM, Vokes NI, Guarente L, Vander Heiden MG, Stephanopoulos G (2013) Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nat Commun 4:2236
Furtado LM, Somwar R, Sweeney G, Niu W, Klip A (2002) Activation of the glucose transporter GLUT4 by insulin. Biochem Cell Biol 80:569–578
Ghorbaniaghdam A, Henry O, Jolicoeur M (2014) An in-silico study of the regulation of CHO cells glycolysis. J Theor Biol 357:112–122
Hao W, Chang CP, Tsao CC, Xu J (2010) Oligomycin-induced bioenergetic adaptation in cancer cells with heterogeneous bioenergetic organization. J Biol Chem 285:12647–12654
Hardie DG, Ashford ML (2014) AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 29:99–107
He Q, Wang M, Petucci C, Gardell SJ, Han X (2013) Rotenone induces reductive stress and triacylglycerol deposition in C2C12 cells. Int J Biochem Cell Biol 45:2749–2755
Ho RC, Fujii N, Witters LA, Hirshman MF, Goodyear LJ (2007) Dissociation of AMP-activated protein kinase and p38 mitogen-activated protein kinase signaling in skeletal muscle. Biochem Biophys Res Commun 362:354–359
Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K (2011) Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60:766–774
Kayali AG, Austin DA, Webster NJ (2000) Stimulation of MAPK cascades by insulin and osmotic shock: lack of an involvement of p38 mitogen-activated protein kinase in glucose transport in 3T3-L1 adipocytes. Diabetes 49:1783–1793
Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ (2007) Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J 403:473–481
Kotliar N, Pilch PF (1992) Expression of the glucose transporter isoform GLUT 4 is insufficient to confer insulin-regulatable hexose uptake to cultured muscle cells. Mol Endocrinol 6:337–345
Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ (2006) Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55:2067–2076
Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A (2005) BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo. J Biol Chem 280:19472–19479
Lali FV, Hunt AE, Turner SJ, Foxwell BM (2000) The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J Biol Chem 275:7395–7402
Lanna A, Henson SM, Escors D, Akbar AN (2014) The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol 15(10):965–972
Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR 3rd, Young LH (2005) AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res 97:872–879
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525
Locasale JW, Cantley LC (2011) Metabolic flux and the regulation of mammalian cell growth. Cell Metab 14:443–451
Manasek FJ, Burnside B, Stroman J (1972) The sensitivity of developing cardiac myofibrils to cytochalasin-B (electron microscopy-polarized light-Z-bands-heartbeat). Proc Natl Acad Sci U S A 69:308–312
Michelle Furtado L, Poon V, Klip A (2003) GLUT4 activation: thoughts on possible mechanisms. Acta Physiol Scand 178:287–296
Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ (2001) AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50:921–927
Newhouse K, Hsuan SL, Chang SH, Cai B, Wang Y, Xia Z (2004) Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci 79:137–146
Novellasdemunt L, Bultot L, Manzano A, Ventura F, Rosa JL, Vertommen D, Rider MH, Navarro-Sabate A, Bartrons R (2013) PFKFB3 activation in cancer cells by the p38/MK2 pathway in response to stress stimuli. Biochem J 452:531–543
Ozcan S, Dover J, Johnston M (1998) Glucose sensing and signaling by two glucose receptors in the yeast Saccharomyces cerevisiae. EMBO J 17:2566–2573
Pelletier A, Joly E, Prentki M, Coderre L (2005) Adenosine 5'-monophosphate-activated protein kinase and p38 mitogen-activated protein kinase participate in the stimulation of glucose uptake by dinitrophenol in adult cardiomyocytes. Endocrinology 146:2285–2294
Pesce MA, Bodourian SH, Nicholson JF (1975) Rapid kinetic measurement of lactate in plasma with a centrifugal analyzer. Clin Chem 21:1932–1934
Rasmussen H, Nielsen R (1968) An improved analysis of adenosine triphosphate by the luciferase method. Acta Chem Scand 22:1745–1756
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017
Somwar R, Kim DY, Sweeney G, Huang C, Niu W, Lador C, Ramlal T, Klip A (2001) GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem J 359:639–649
Somwar R, Perreault M, Kapur S, Taha C, Sweeney G, Ramlal T, Kim DY, Keen J, Cote CH, Klip A, Marette A (2000) Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle: potential role in the stimulation of glucose transport. Diabetes 49:1794–1800
Susztak K, Raff AC, Schiffer M, Bottinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55:225–233
Turban S, Beardmore VA, Carr JM, Sakamoto K, Hajduch E, Arthur JS, Hundal HS (2005) Insulin-stimulated glucose uptake does not require p38 mitogen-activated protein kinase in adipose tissue or skeletal muscle. Diabetes 54:3161–3168
Wagenknecht B, Lieberman M (1991) Adenine nucleotide degradation in cultured chick heart muscle cells. Mol Cell Biochem 107:119–125
Xi X, Han J, Zhang JZ (2001) Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem 276:41029–41034
Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4:33–41
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, C.G., Burkholder, T.J. Activation of p38 in C2C12 myotubes following ATP depletion depends on extracellular glucose. J Physiol Biochem 71, 253–265 (2015). https://doi.org/10.1007/s13105-015-0406-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0406-z