Abstract
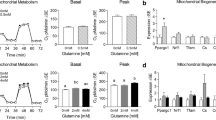
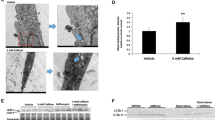
Activation of 5′ adenosine monophosphate-activated protein kinase (AMPK) with aminoimidazole carboxamide ribonucleotide (AICAR) increases skeletal muscle glucose uptake and fatty acid oxidation. The purpose of these experiments was to utilize AICAR to enhance palmitate consumption by mitochondria in cultured skeletal muscle cells. In these experiments, we treated C2C12 myotubes or adult single skeletal muscle fibers with varying concentrations of AICAR for different lengths of time. Surprisingly, acute AICAR exposure at most concentrations (0.25–1.5 mM), but not all (0.1 mM), modestly inhibited oxygen consumption even though AICAR increased AMPK phosphorylation. The data suggest that AICAR inhibited oxygen consumption by the cultured muscle in a non-specific manner. The results of these experiments are expected to provide valuable information to investigators interested in using AICAR in cell culture studies.





Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl CoA-carboxylase
- AICAR:
-
Aminoimidazole carboxamide ribonucleotide
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- DM:
-
Differentiation media
- ETC:
-
Electron transport chain
- FCCP:
-
Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- FDB:
-
Flexor digitorum brevis
- MB:
-
Extracellular flux measurement buffer
- OCR:
-
Oxygen consumption rates
- PA:
-
Sodium palmitate
- SRC:
-
Spare respiratory capacity
References
Bai J, Cederbaum AI (2000) Overexpression of catalase in the mitochondrial or cytosolic compartment increases sensitivity of HepG2 cells to tumor necrosis factor-alpha-induced apoptosis. J Biol Chem 275:19241–19249
Beylkin DH, Allen DL, Leinwand LA (2006) MyoD, Myf5, and the calcineurin pathway activate the developmental myosin heavy chain genes. Dev Biol 294:541–553
Brown LD, Schneider MF (2002) Delayed dedifferentiation and retention of properties in dissociated adult skeletal muscle fibers in vitro. In Vitro Cell Dev Biol An 38:411–422
Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E (2006) Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metabolism 3:403–416
Flanagan WF, Holmes EW, Sabina RL, Swain JL (1986) Importance of purine nucleotide cycle to energy production in skeletal muscle. Am J Physiol 251:C795–C802
Fogarty S, Hardie DG (2010) Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta 1804:581–591
Foley JM, Adams GR, Meyer RA (1989) Utility of AICAr for metabolic studies is diminished by systemic effects in situ. Am J Physiol 257:C488–C494
Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD (2009) Quantitative microplate-based respirometry with correction for oxygen diffusion. Annal Chem 81:6868–6878
Guigas B, Taleux N, Foretz M, Detaille D, Andreelli F, Viollet B, Hue L (2007) AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem J 404:499–507
Hardie DG, Carling D, Carlson M (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell. Annu Rev Biochem 67:821–855
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metabolism 11:554–565
Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ (1998) Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes 47:1369–1373
Howlett RA, Kindig CA, Hogan MC (2007) Intracellular PO2 kinetics at different contraction frequencies in Xenopus single skeletal muscle fibers. J Appl Physiol 102:1456–1461
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104:12017–12022
Lanner JT, Georgiou DK, Dagnino-Acosta A, Ainbinder A, Cheng Q, Joshi AD, Chen Z, Yarotskyy V, Oakes JM, Lee CS, Monroe TO, Santillan A, Dong K, Goodyear L, Ismailov II, Rodney GG, Dirksen RT, Hamilton SL (2012) AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nature Med 18:244–251
Lau GY, Richards JG (2011) AMP-activated protein kinase plays a role in initiating metabolic rate suppression in goldfish hepatocyte. J Comp Physiol B, Biochem, Sys Environ Physiol 181:927–939
Lee-Young RS, Griffee SR, Lynes SE, Bracy DP, Ayala JE, McGuinness OP, Wasserman DH (2009) Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem 284:23925–23934
McAllister RM, Terjung RL (1990) Acute inhibition of respiratory capacity of muscle reduces peak oxygen consumption. Am J Physiol 259:C889–C896
Merrill GF, Kurth EJ, Hardie DG, Winder WW (1997) AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol 273:E1107–E1112
Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134:405–415
Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA (2010) Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. (46): pii: 2511
O'Neill HM, Maarbjerg SJ, Crane JD, Jeppesen J, Jorgensen SB, Schertzer JD, Shyroka O, Kiens B, van Denderen BJ, Tarnopolsky MA, Kemp BE, Richter EA, Steinberg GR (2011) AMP-activated protein kinase (AMPK) beta1beta2 muscle null mice reveal an essential role for AMPK in maintaining mitochondrial content and glucose uptake during exercise. Proc Natl Acad Sci U S A 108:16092–16097
Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D (1998) Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J 17:1688–1699
Raney MA, Yee AJ, Todd MK, Turcotte LP (2005) AMPK activation is not critical in the regulation of muscle FA uptake and oxidation during low-intensity muscle contraction. Am J Physiol Endocrinol Metab 288:E592–E598
Robinson DM, Ogilvie RW, Tullson PC, Terjung RL (1994) Increased peak oxygen consumption of trained muscle requires increased electron flux capacity. J Appl Physiol 77:1941–1952
Schuh RA, Jackson KC, Khairallah RJ, Ward CW, Spangenburg EE (2012) Measuring mitochondrial respiration in intact single muscle fibers. Am J Physiol Regul Integ Comp Physiol 302:R712–R719
Segalen C, Longnus SL, Baetz D, Counillon L, Van Obberghen E (2008) 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside reduces glucose uptake via the inhibition of Na+/H+ exchanger 1 in isolated rat ventricular cardiomyocytes. Endocrinology 149:1490–1498
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89:1025–1078
Wang CZ, Wang Y, Di A, Magnuson MA, Ye H, Roe MW, Nelson DJ, Bell GI, Philipson LH (2005) 5-Amino-imidazole carboxamide riboside acutely potentiates glucose-stimulated insulin secretion from mouse pancreatic islets by KATP channel-dependent and -independent pathways. Biochem Biophys Res Commun 330:1073–1079
Winder WW, Hardie DG (1996) Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 270:E299–E304
Wohlers LM, Sweeney SM, Ward CW, Lovering RM, Spangenburg EE (2009) Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J Cell Biochem 107:171–178
Zheng D, Perianayagam A, Lee DH, Brannan MD, Yang LE, Tellalian D, Chen P, Lemieux K, Marette A, Youn JH, McDonough AA (2008) AMPK activation with AICAR provokes an acute fall in plasma [K+]. Am J Physiol: Cell physiol 294:C126–C135
Acknowledgments
The authors wish to thank Dr(s) David Thomson, Andy Philp, Keith Baar, and John Thyfault for insightful discussions. The work was supported by grants from the National Institutes of Health AR059913 (EES), Baltimore Diabetes Research Training Center Grant (EES) (P60DK079637), Rehabilitation R&D REAP and Biomedical R&D CDA-02 from the VA Research Service (RAS). The funding sources play no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spangenburg, E.E., Jackson, K.C. & Schuh, R.A. AICAR inhibits oxygen consumption by intact skeletal muscle cells in culture. J Physiol Biochem 69, 909–917 (2013). https://doi.org/10.1007/s13105-013-0269-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0269-0