Atazanavir is a protease inhibitor with relatively few side effects and an advantageous lipid profile. Since its approval, 9 cases of acute renal failure associated with this drug have been described. In 7 of these cases, atazanavir was associated with tenofovir1–4, a drug with well-recognized kidney-related side effects. In an additional case, renal failure was associated with the combination of atazanavir and amiodarone, both CYP3A4 inhibitors, and simvastatin, which requires this isoenzyme for metabolism.5 Atazanavir use alone, without tenofovir or CYP3A4-inhibiting drugs, has been linked to renal failure in only one case6.
We report the case of an HIV-positive man with chronic renal insufficiency, who developed acute renal failure that was likely related to atazanavir administration, without concurrent tenofovir. A 37-year-old former intravenous drug user had been diagnosed of HIV infection in 1988. He had HCV, HBV and HDV infection with cirrhosis (Child-Pugh class B, score 9), and had experienced wasting, HIV encephalopathy, and numerous respiratory infections, including Pneumocystis jiroveci pneumonitis. There was no evidence of hypertension, diabetes mellitus, or dyslipidemia. He had followed many different antiretroviral therapies (ART) with varying degrees of adherence and frequent gastrointestinal side effects. His medical records included an abacavir hypersensitivity reaction. The latest ART, guided by genotype sensitivity tests, included lopinavir/ritonavir 400mg/100mg capsules bid, didanosine 250mg capsules qd, and lamivudine 300mg tablets qd, and achieved an undetectable viral load (<50copies/mL) with a CD4+ count of 310cells/μL. At a time when compliance with ART was suboptimal, he experienced recurrent episodes of gross hematuria with high IgA levels (988mg/dL) that were considered a consequence of IgA nephropathy. Starting with the onset of hematuria, the patient's renal function deteriorated over the next 12 months. Plasma creatinine increased from 1.2 to 2mg/dL (abbreviated MDRD adjusted for body surface, 41mL/min). Renal ultrasound showed no renal parenchymal or urinary tract changes. To improve adherence to ART, atazanavir capsules at a dose of 300 qd, boosted with 100mg ritonavir, were substituted for bid lopinavir/ritonavir, and didanosine and lamivudine doses were adjusted for renal failure (125mg capsules qd and 150mg capsules qd, respectively). Ritonavir had to be withdrawn because of digestive intolerance and atazanavir dose was increased to 400mg qd.
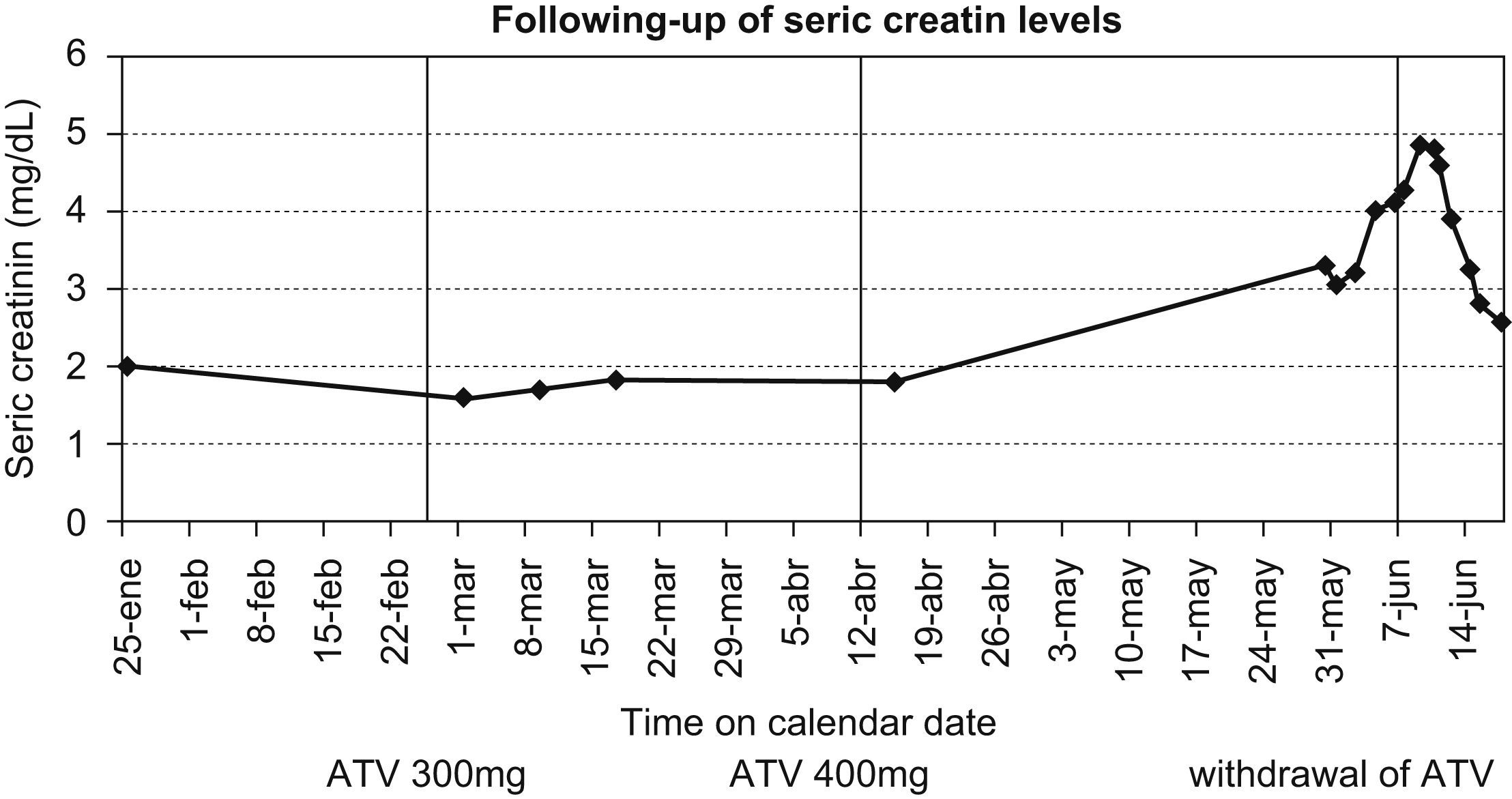
After 45 days of treatment (atazanavir 400mg qd, didanosine 125mg/day and lamivudine 150mg/day), the patient developed a febrile illness with respiratory symptoms, and worsening of renal function. He again showed gross hematuria with mild proteinuria (25mg/dL), and 1-4 leucocytes/high power field in the urinary sediment; there was no oliguria, urinary frequency, or dysuria. At admission, he had low blood pressure (90/60mm Hg) and fever (38°C), and showed mild wheezing, hepatosplenomegaly, and slight ankle edema. The most salient laboratory findings were serum creatinine 3.29mg/dL (body surface-adjusted abbreviated MDRD, 18mL/min), venous blood pH 7.38, CO3H− 21.8mmol/L, potassium 5.5mEq/L, hemoglobin 8.3g/dL and 89000platelets/μL. Protein level in 24-hour urine was 0.16g. Urine cytology, blood and urine cultures, and urine examination for acid-fast bacilli were negative. There was no peripheral blood eosinophilia or eosinophiluria. Renal ultrasound showed hyperechoic cortices, suggesting medical renal disease. Laboratory evaluation disclosed type III cryoglobulinemia (cryocrit 4.3%), with positive HCV-PCR. ANCA and ANA were negative. There was no complement consumption and serum IgA remained increased. Despite correction of functional factors and defervescence of fever, plasma creatinine was persistently high, peaking at 4.85mg/dL (abbreviated MDRD, 21mL/min) 3 days after atazanavir withdrawal. Thereafter, serum creatinine levels dropped, reaching 2.56mg/dL at discharge.
Because of the improvement in renal function, resolution of hematuria after atazanavir withdrawal, and the risk of renal biopsy in a patient with thrombocytopenia and cirrhosis, biopsy was deemed unnecessary. After discharge, ART with lopinavir/ritonavir (400mg/100mg bid), didanosine (125mg qd) and lamivudine (150mg qd) was restarted, with no worsening of renal function. One month after hospital discharge, neuropathy developed, and didanosine was withdrawn; tenofovir in tablets at a dose of 300mg every other day was introduced, with no renal function deterioration or hypophosphatemia at follow-up. Five months after atazanavir withdrawal, serum creatinine was 1.58mg/dL (abbreviated MDRD, 31mL/min). Renal function remained stable at this level until the death of the patient 18 months later, due to pneumonia. The patient never passed a stone and calculi were not seen in image studies.
Renal dysfunction is common in HIV-positive patients, increasing the morbidity and mortality of this population. Acute tubular necrosis, due to hypovolemia caused by vomiting, diarrhea, sepsis, hypoxia, nephrotoxic drugs used in the treatment of opportunistic infections, and ART, itself, are the main causes of acute renal failure in HIV-positive patients. Among these treatments, antimicrobials are most commonly involved in renal complications, whereas antiretroviral drugs are seldom implicated. Only indinavir and tenofovir have been clearly associated with renal failure. In a recent review, Röling et al,7 described the relationship between antiretrovirals and renal disease. The most commonly implicated PIs are ritonavir, saquinavir, nelfinavir and indinavir, this last drug ranking first in adverse renal effects. Other PIs, such as amprenavir, fosamprenavir, and lopinavir have not been related to renal damage. Among the nucleotide and nucleoside analogue reverse transcriptase inhibitors (NRTIs), tenofovir is the most nephrotoxic drug. Renal toxicity due to NRTIs is generally rare, and very limited data about non-nucleoside inhibitors (NNRTI) are available. Nevirapine and efavirenz have a favorable renal profile8.
In our patient, blood and urine cultures were negative, which decreases the likelihood of an infectious cause of renal impairment. A role of HCV-associated cryoglobulinemia cannot be excluded, though the cryocrit was low.
To assess possible side effects undetected in the development phase of a drug, post-marketing surveillance is crucial. We describe a patient with renal insufficiency that was temporally related to the administration of atazanavir. To our knowledge, only one similar case has been reported. Brewster et al.6 described a patient treated with abacavir, 300mg bid, lamivudine 150mg bid, and atazanavir, 400mg qd. The patient was diagnosed of acute interstitial nephritis, and a renal biopsy showed tubulointerstitial nephritis superimposed on glomerulosclerosis. Atazanavir was considered the most probable cause, since it had been introduced 1 month before the earliest signs of renal failure, while the other drugs had been present at stable doses for 5 months.
Our patient developed acute renal failure on chronic renal insufficiency, the latter probably due to IgA-nephropathy. We could not ascertain the precise mechanism of renal failure because a renal biopsy was not performed. Acute interstitial nephritis, HIV-associated glomerulonephritis, HIV-associated nephropathy, HCV-associated membranoproliferative glomerulonephritis, and direct nephrotoxic effects were considered in the differential diagnosis. These, in turn, can be aggravated by acute tubular necrosis due to infectious disease or functional factors. Given the response to atazanavir withdrawal, we believe this drug was linked to renal worsening. The patient was receiving lamivudine and didanosine as well. Several cases of Fanconi-type renal dysfunction related to lamivudine and didanosine have been reported9,10, but they are rare and this patient had been previously treated with these drugs without adverse renal effects. At full therapeutic doses of 800–1200mg daily7, ritonavir has also been implicated in renal dysfunction, but our patient only received a daily booster dose of 100mg, and the drug had been withdrawn before the signs of renal failure appeared.
As shown in fig. 1, renal function, as measured by serum creatinine levels, began to worsen after ritonavir withdrawal, coinciding with the increased dose of atazanavir. Serum creatinine peaked 3 days after atazanavir withdrawal, and thereafter, the values began to return to baseline levels. At a dose of 400mg per day, atazanavir has a half-life of 5 to 8h; thus the drug clears in 1–2 days, a period that was temporally related to the drop in creatinine values.