Abstract
Ocoxin is a nutritional supplement that has been shown to exert antioxidant and immunomodulatory responses in patients with chronic hepatitis C. The present work aimed to determine the effects of Ocoxin on activated hepatic stellate cells (HSC), the cell type mainly responsible for collagen deposition in the fibrotic liver. Ocoxin was found to reduce the survival of a cell line of immortalized non-tumoral rat HSC in a dose–response fashion and to diminish collagen type I levels. This latter effect was observed even at doses not affecting cell survival, pointing to an antifibrogenic action for the supplement. The decrease in viability exerted by Ocoxin on HSC correlated with an increase in histone-associated fragments in the cytoplasm and with increased activity of caspase-3, indicating the induction of apoptosis. To determine the molecular mechanisms mediating Ocoxin-induced apoptosis, the activation of members of the MAPK family was analyzed. Incubation of HSC with Ocoxin caused a transient and dramatic enhancement on ERK, JNK, and p38 MAPK phosphorylation levels. Using specific inhibitors for these enzymes, p38 MAPK was identified as a key mediator of the apoptotic effect of Ocoxin on HSC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatic fibrosis is a pathological condition that can be triggered by a wide range of agents and is characterized by excessive accumulation of extracellular matrix proteins in the liver. Progressive fibrosis can eventually result in cirrhosis, liver failure, or hepatocellular carcinoma, and it is considered the hallmark of chronic liver injury [1, 5]. Several cell types regulated by a complex net of factors are involved in the development of liver fibrosis. Among them, activated hepatic stellate cells (HSC) are the cell type mainly responsible for extracellular matrix deposition and therefore a key target for antifibrogenic strategies [29]. Oxidative stress due to agents like alcohol or the profibrogenic cytokine transforming growth factor-beta (TGF-β) or caused by infiltrating immune cells has been shown to mediate the development of hepatic fibrosis and to directly contribute to collagen production by HSC [2, 26]. A relevant number of studies have evaluated the effects of different antioxidants, either by themselves of combined with other agents, in the progression of liver fibrosis, showing in most instances moderate but significant beneficial effects [15, 35], and in some cases even detrimental outcomes [21].
Ocoxin is a nutritional supplement which contains agents that have been reported to cause antioxidant and/or hepatoprotective actions, such as glycyrrhizic acid [16, 19], ascorbic acid [8], or epigallocatechin [28]. Clinical studies carried out in patients with chronic hepatitis C [9, 32] and cirrhosis [34] have demonstrated Ocoxin treatment to improve oxidative stress and immunological parameters as well as to diminish the extent of liver fibrosis. In patients with nonalcoholic fatty liver disease (NFLD), the administration of Ocoxin resulted in an improvement in some histological markers of steatosis and inflammation [33]. Additionally, the administration of this nutritional supplement inhibited the proliferation of human hepatocellular carcinoma cell lines and reduced tumor progression in animal models of liver cancer [6]. Moreover, the induction of apoptosis has also been described in metastatic liver lesions derived from an experimental animal model of colorectal carcinoma treated with Ocoxin [17]. To date, the mechanisms through which Ocoxin exerts these effects have not been fully established. Taking into account the key role played by HSC in the development of liver fibrosis, the aim of the present study was to determine the potential effects of Ocoxin on HSC and to analyze the molecular mechanisms involved.
Materials and methods
Reagents
Cell culture reagents were obtained from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). 5–6-Chloromethyl-2′,7′-dichlorohydrofluorescein diacetate (CM-H2DCFDA) was from Molecular Probes (Eugene, OR). SB203580 and PD98059 were purchased from Calbiochem® (Germany). L-JNKI1 was from ALEXIS® Biochemicals (Lausen, Switzerland). General chemical reagents were purchased from Sigma Aldrich (Merck KGaA, Darmstadt, Germany) unless otherwise specified. Cell culture plastics were obtained from Corning (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Ocoxin was from Catalysis S.L. (Madrid, Spain). As shown in Table 1, Ocoxin is a formulation that includes the components mentioned in the list.
Cell culture and materials
The experiments were performed using the HSC line CFSC-2G. This non-tumoral cell line was obtained after spontaneous immortalization of HSC isolated from a rat CCl4-cirrhotic liver and is characterized by low basal levels of expression of type I collagen genes and by the presence of mRNA for nestin and α-SMA. Therefore, it can be considered as a “transitional” HSC, in which the activation process is already initiated. Cells were cultured in MEM supplemented with 10% fetal bovine serum (FBS) and non-essential amino acids for 36 h after which the medium was replaced with a serum-free medium. Treatments were carried out 12 h later. HSC were treated with Ocoxin for the indicated times and dilutions (in culture media). In some experiments, cells were pretreated for 30 min with either 10 μM SB203580, PD98059, or L-JNKI1. The specificity of these inhibitors at concentrations used in the present study has been demonstrated in previous reports [31]. Protein inhibitors were initially dissolved in dimethyl sulfoxide (DMSO) (“vehicle”; ≥ 99.9%, Merck, Darmstadt, Germany). The same amount of DMSO (vehicle) was added to control cells to discard possible effects related to the vehicle. The LX-2 cell line, an immortalized human hepatic stellate cell line (Merck, Darmstadt, Germany) was cultured in high glucose DMEM supplemented with 2% heat-inactivated FBS and 1% penicillin/streptomycin (5000 U/mL) (Lonza, Basel, Switzerland). The human hepatoma cell lines HuH7 and HepG2 were obtained from the American Type Culture Collection (Rockville, MD) and cultured in DMEM supplemented with 10% FBS, l-glutamine, and antibiotics. Thirty-six hours after cells were plated, the medium was replaced by DMEM supplemented without FBS, and treatments were carried out 12 h later. Freshly isolated hepatocytes were obtained by liver collagenase perfusion from male Wistar rats. Isolated hepatocytes were resuspended and plated in MEM supplemented with 10% FBS, antibiotics, and non-essential amino acids. They were cultured in plates for two hours. After this time, the culture media was replaced by freshly culture media in order to remove dead cells. The hepatocytes were then cultured for 24 h, and afterwards, the culture media was replaced by a medium without FBS, and treatments were carried out 12 h later.
Neutral red assay
Cell viability was analyzed using neutral red (NR, 3-amino-7-dimethylamino-2-methylphenazin), a weak cationic stain which is actively concentrated by viable cells and accumulates in lysosomes. For NR assay 4 × 104 CFSC-2G, LX2, hepatocytes, HUH7, or HepG2 cells were seeded in a 96-well plate and treated during 24 h with the indicated dilutions of Ocoxin. Then, 50 μl of NR 1 mg/mL:NaCl 1.8% (1:1) was added and cells were incubated for 90 min, at 37 °C in a 5% CO2 atmosphere. Cells were washed twice with PBS and the vital dye incorporated by viable cells was released by adding 100 μl of NaH2PO4 0.05 M and 50% ethanol. The absorbance of the samples was measured at 540 nm and referred to the absorbance of untreated cells. Values are mean ± SD of at least triplicate data from three independent experiments.
Determination of oligonucleosomal (histone-associated) DNA fragments
The presence of soluble histone-DNA complexes was measured by the Cell Death Detection Assay (Roche® Life Science Products, Merck KGaA, Darmstadt, Germany). For this assay, HSC were seeded on 96-well plates at a density of 4 × 104 cells/well. Cell death ELISA assays was performed according to the manufacturer’s instructions. Specific enrichment of mono- and oligonucleosomes released into the cytoplasm (enrichment factor, EF) was calculated as the ratio between the absorbance values of the samples obtained from treated and control cells.
Measurement of caspase-3 activity
The caspase 3 colorimetric assay kit (Sigma Aldrich Merck KGaA, Darmstadt, Germany) was used to detect caspase-3 activity. The assay is based on the spectrophotometric detection of the chromophore p-nitroaniline (pNA) which is released after cleavage of the substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase-3. The presence of pNA was measured with an ELISA plate reader (Multiskan Ex, Thermo Electron Corporation) reading the absorbance at 405 nm. Absorbance values of treated samples were compared with those of control samples to determine the increase of caspase-3 activity. For this assay, 6 × 105 cells were seeded in 60-mm plates and treated for 6 h with Ocoxin at different dilutions.
Measurement of intracellular reactive oxygen species (ROS) levels
ROS were measured using the fluorescent probe CM-H2DCFDA. For these experiments, HSC were grown in MEM without phenol red. For time course studies, HSC were plated to sub-confluence in 60-mm culture dishes, treated at different times with Ocoxin and then incubated for 20 min with 5 μM CM-H2DCFDA at room temperature. Fluorescence was analyzed in a Cytofluor 2350 (excitation at 485 nm, emission at 530 nm).
Western blot analysis
HSC were treated as described above and proteins extracted in RIPA buffer (25 mM Tris–HCl, 150 mM NaCl, 0.1% w/v SDS, 1% w/v sodium deoxycholate, 1% v/v IGEPAL). The protein concentration of the resultant samples was determined by BCA (bicinchoninic acid assay, Sigma Aldrich Merck KGaA, Darmstadt, Germany). For immunoblotting analysis, equal amounts of protein were electrophoresed on SDS–polyacrylamide gels and transferred on membranes. Membranes were incubated with a specific antibody to phospho-ERK (Promega), phospho-JNK, phospho-p38 MAPK, type I collagen (Rockland Inmunochemicals) or actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with the secondary antibody conjugated to horseradish peroxidase (Promega), immunoreactive proteins were detected by an enhanced chemiluminescent system (ECL, Amersham International plc, Little Chalfont, UK).
Statistical analysis
Data were analyzed using one-way ANOVA to determine differences between all independent groups. When significant differences were obtained (p < 0.05), differences between groups were tested using Dunnett’s multiple comparisons test. Graphs were generated using GraphPad Prism 9 (GraphPad, San Diego, CA, USA).
Results
Ocoxin diminishes cell viability of HSC
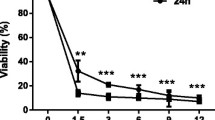
The first experiments aimed to determine the effect of Ocoxin on cell survival of the HSC line CSFC-2G. Cell cultures were exposed for 24 h to increasing dilutions of Ocoxin, ranging from 1:10 to 1:1000. The morphological effects of Ocoxin on HSC were evaluated by light microscopy for the 1:50 and 1:100 dilutions. As shown in Fig. 1A, Ocoxin caused, in a dose–response fashion, a highly refringent and round-shaped cells appearance. Cell viability assay, using neutral red, showed a correlation of morphological changes and loss of cell viability, which was significant for 1:10–1:100 dilutions (Fig. 1B).
Analysis of HSC viability in response to Ocoxin. HSC were treated for 24 h with the indicated dilutions of Ocoxin. A Morphology by light microscopy of control and Ocoxin-treated HSC. Original magnification × 200. B Cell viability in response to Ocoxin determined by neutral red assay. Control represents non-treated cells. Each bar represents the mean ± SD of percentage viability fold change compared to control, of triplicate data from four independent experiments (*p < 0.05, ***p < 0.001, vs control)
The reduced viability of HSC was confirmed in the human HSC cell line LX-2 and the hepatoma cell lines HepG2 and HuH7, employing the same dilutions of the nutritional supplement. Noteworthy, only the lowest dilution of Ocoxin significantly affected the cell viability of primary hepatocytes (Fig. 2).
Cell viability in response to Ocoxin treatment for 24 h determined by a neutral red assay in; A human hepatic stellate cells LX-2. B Normal rat hepatocytes. C Human hepatoma cell line HepG2. D Human hepatoma cell line HuH7. Control represents non-treated cells. Each bar represents the mean ± SD of percentage viability fold change compared to control of triplicate data from four independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001, vs control)
Ocoxin reduces the production of type I collagen in HSC
The analysis of the effect of Ocoxin on the production of type I collagen in HSCs was carried out by Western blot determination of its precursor form procollagen α1 (I) levels. To this purpose, a 24-h treatment was conducted with different dilutions of Ocoxin, including a dilution with an effect on cell viability (1:100) and dilutions with no effect on cell viability (1:500 and 1:1000). As shown in Fig. 3, collagen type I levels were decreased in all cases, suggesting an antifibrogenic effect even at low doses that did not affect cell viability.
Effect of Ocoxin on procollagen α1(I) levels in HSCs. HSCs were incubated with the indicated dilutions of Ocoxin for 24 h. Protein extracts were analyzed with Western blot using specific antibodies for procollagen α1 (I) and β-actin. β-Actin protein levels were used as the loading control. Results are representative of at least three independent experiments. (*p < 0.05, **p < 0.01, vs control)
Ocoxin induces apoptosis in HSC
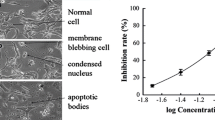
The presence of histone-associated oligonucleosomal fragments in the cytoplasm reflects the extent of DNA fragmentation and nuclear disruption that are characteristic of apoptosis. To determine whether the decrease in cell survival caused by Ocoxin was due to the induction of apoptosis, cytoplasmic levels of oligonucleosomal fragments were analyzed by ELISA in HSC treated with several dilutions of Ocoxin. A significant enhancement on oligonucleosomal fragment content was observed in response to treatment with all the dilutions, being higher for 1:10 to 1:100 dilutions (Fig. 4A).
Analysis of HSC apoptosis in response to Ocoxin. A Determination of oligonucleosomal fragments in cytoplasmic extracts from HSC treated with Ocoxin. Oligonucleosomal fragments content was determined by ELISA and expressed as enrichment factor (EF), as described in Materials and methods. B Caspase-3 activity levels were analyzed in HSC treated with Ocoxin for 6 h, using a commercial colorimetric kit, as indicated in the “Materials and methods” section. Each bar represents the mean + SD of quadruplicate determinations from at least two independent experiments (***p < 0.001, vs control)
Caspases are a family of cysteine proteases which are essential mediators of the apoptotic process. Caspase-3 is an ultimate effector of caspase-mediated apoptosis. To study whether Ocoxin-induced apoptosis is caspase-mediated, the activity of this enzyme was measured with a commercial kit in HSCs incubated with the supplement for 6 h. As observed in Fig. 4B, treatment of the HSCs with different dilutions of Ocoxin caused an increase of caspase-3 activity, suggesting a caspase-dependent cell death induced by Ocoxin.
In order to determine the possible involvement of reactive oxygen species (ROS) in the molecular mechanisms that could mediate the apoptotic effect of Ocoxin, intracellular levels of ROS were analyzed using the fluorogenic probe CM-H2DCFDA in HSC treated for 1–6 h with these different dilutions of this nutritional supplement (Supplementary Figure S1). However, only a slight and transient increase in intracellular levels of ROS was observed 6 h after treatment, pointing to a ROS-independent apoptotic mechanism.
Role of members of the MAPK family on apoptosis of HSC induced by Ocoxin
Once established the type of cell death induced by Ocoxin, we next evaluated the involvement of the main MAPK in the apoptotic effect of the nutritional supplement. Time course treatments using a 1:100 dilution of Ocoxin were carried out and the phosphorylated forms of ERK, JNK, and p38MAPK were detected by Western blot analysis using specific antibodies. As shown in Fig. 5, increased phosphorylation of ERK, JNK, and p38MAPK was observed reaching maximum values 5–15 min after treatment.
Analysis of ERK, JNK, and p38MAPK signaling pathways in HSC treated with Ocoxin. HSC were treated with Ocoxin 1:100 dilution for 5–60 min and Western blot analysis for the phosphorylated active forms of ERK, JNK, and p38MAPK was carried out using specific antibodies. β-Actin protein levels were used as the loading control. Results are representative of at least three independent experiments
To establish whether activation of the enzymes analyzed was involved in the apoptotic action of Ocoxin, HSC were pretreated for 30 min with pharmacological inhibitors for p38MAPK (SB203580), JNK (L-JNKI1), or ERK (PD098059, inhibitor of MEK1) prior incubation with 1:100 dilution of Ocoxin, and the extent of apoptotic cell death was determined as above. Inhibition of JNK did not alter the cytosolic levels of oligonucleosomal fragments enhanced by Ocoxin, suggesting this enzyme is not involved in the apoptotic response. Pretreatment with the inhibitor for ERK activation, on the other hand, had a potentiating effect on the apoptotic action of Ocoxin, indicating that ERK could have an antiapoptotic or proliferative role on HSC. Finally, the inhibitor for p38 MAPK significantly prevented the apoptotic effect of Ocoxin, demonstrating p38 MAPK is involved in the apoptotic response (Fig. 6).
Role of p38 MAPK, JNK, and ERK signaling on HSC apoptosis induced by Ocoxin. HSC were pretreated for 30 min with 10 μM SB203580 (p38MAPK inhibitor), L-JNKI1 (JNK inhibitor) or PD098059 (MEK1 inhibitor), and treated with 1:100 dilution of Ocoxin. Determination of oligonucleosomal fragments in cytoplasmic extracts was carried out by ELISA and expressed as enrichment factor (EF), as described in the “Materials and methods” section. Each bar represents the mean + SD of quadruplicate determinations from at least three independent experiments (**p < 0.01, ***p < 0.001, a, vs control; b, vs Ocoxin-treated cells)
Discussion
Liver fibrosis is considered the result of the dysregulation caused by chronic injury of an otherwise transient process, the wound healing response, and therefore a pathological situation that could be eventually reversible. In fact, either fibrotic or cirrhotic regression has been described in most chronic liver diseases, including autoimmune hepatitis, NASH, and viral hepatitis [13]. Together with other processes, such as cellular senescence [14], clearance of activated HSC through apoptosis has been demonstrated to contribute to fibrosis regression both in animal models [11] and clinical studies [20]. These observations confirm previous results demonstrating the contribution of enhanced HSC survival to progressive fibrosis [22, 24]. In the present study, Ocoxin was found to present antifibrotic activity as it induced apoptosis and reduced collagen production in a cell line of immortalized rat HSC (Fig. 7). The cell line used (2G-CFSC) can be considered as a “transitional” HSC, in which the activation process is already initiated [31] and therefore a good model for early fibrosis. A pro-apoptotic effect of Ocoxin on activated HSC could explain some of the beneficial outcomes found in clinical studies carried out with hepatitis C patients, in which treatment with Ocoxin either by itself [9] or combined with interferon alpha-2b and ribavirin caused a reduction of fibrosis [32]. Regarding NAFLD, Ocoxin seemed to have a more relevant effect on inflammation and in histological markers of steatosis rather than in fibrosis [33].
Endogenous production of reactive oxygen species (ROS) is altered in HSC in response to TGF-β, acetaldehyde [10], or leucine [25], leading through different mechanisms to an increased fibrogenic activity. On the other hand, prooxidant agents like menadione or SIN-1 have been described to induce apoptosis of HSC, an effect that was prevented by pretreatment with antioxidants [18]. Although ROS can regulate HSC biology and Ocoxin has been shown to improve oxidative parameters in vivo, the apoptotic effect of this compound on HSC does not seem to be mediated by alterations in ROS intracellular content since only some slight changes at certain doses were observed (Supplementary Figure S1). In fact, there is increasing evidence indicating that many beneficial effects of agents with antioxidant potential are due to mechanisms not directly related to ROS scavenging, such as regulation of transcription factors or signaling pathways [4, 27].
Mitogen-activated protein kinase (MAPK) superfamily members p42/p44 MAPK (ERK), and the stress-activated members c-Jun amino-terminal kinase (JNK), and p38 MAPK, are mediators of the cellular responses to stimuli like growth factors or stress signals. More specifically, JNK and p38 MAPK have been shown to exert pro-apoptotic effects in different cell types. We found Ocoxin to induce early and transient activation of ERK, JNK, and p38MAPK. Although both JNK and p38 MAPK have been previously demonstrated to be involved in apoptosis of HSC in response to different agents [7, 12], only p38 MAPK seemed to mediate the effect of Ocoxin since inhibition of this enzyme but not of JNK significantly prevented apoptosis of HCS. Interestingly, p38 MAPK plays also an important role in the profibrogenic response of HSC, mediating the activation process and both basal and TGF-β-induced production of collagen type I [3, 30, 31]. Our results give additional evidence of p38 MAPK being a key mediator of the biology of HSC, involved not only in their fibrogenic activity but also in the molecular mechanisms leading to their programmed cell death.
Data availability
The data that support the findings of this study are available from the corresponding author, Dra María J. Iraburu, upon reasonable request.
References
Acharya P, Chouhan K, Weiskirchen S, Weiskirchen R (2021) Cellular mechanisms of liver fibrosis. Front Pharmacol 12:671640. https://doi.org/10.3389/fphar.2021.671640
Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I (2018) TGF-beta and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci 19(5):1294. https://doi.org/10.3390/ijms19051294
Cao Q, Mak KM, Lieber CS (2002) DLPC decreases TGF-beta1-induced collagen mRNA by inhibiting p38 MAPK in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 283(5):G1051–G1061. https://doi.org/10.1152/ajpgi.00128.2002
Carter CA, Kane CJ (2004) Therapeutic potential of natural compounds that regulate the activity of protein kinase C. Curr Med Chem 11(21):2883–2902. https://doi.org/10.2174/0929867043364090
Dhar D, Baglieri J, Kisseleva T, Brenner DA (2020) Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med (Maywood) 245(2):96–108. https://doi.org/10.1177/1535370219898141
Diaz-Rodriguez E, El-Mallah AM, Sanz E, Pandiella A (2017) Antitumoral effect of Ocoxin in hepatocellular carcinoma. Oncol Lett 14(2):1950–1958. https://doi.org/10.3892/ol.2017.6440
Dunning S, Hannivoort RA, de Boer JF, Buist-Homan M, Faber KN, Moshage H (2009) Superoxide anions and hydrogen peroxide inhibit proliferation of activated rat stellate cells and induce different modes of cell death. Liver Int 29(6):922–932. https://doi.org/10.1111/j.1478-3231.2009.02004.x
Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD (2011) Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol 106(1):71–77. https://doi.org/10.1038/ajg.2010.299
Gomez EV, Perez YM, Sanchez HV, Forment GR, Soler EA, Bertot LC, Garcia AY, del Rosario Abreu Vazquez M, Fabian LG (2010) Antioxidant and immunomodulatory effects of Viusid in patients with chronic hepatitis C. World J Gastroenterol 16(21):2638–47. https://doi.org/10.3748/wjg.v16.i21.2638
Greenwel P, Dominguez-Rosales JA, Mavi G, Rivas-Estilla AM, Rojkind M (2000) Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology 31(1):109–116. https://doi.org/10.1002/hep.510310118
Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP (2001) Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 48(4):548–557. https://doi.org/10.1136/gut.48.4.548
Jameel NM, Thirunavukkarasu C, Wu T, Watkins SC, Friedman SL, Gandhi CR (2009) p38-MAPK- and caspase-3-mediated superoxide-induced apoptosis of rat hepatic stellate cells: reversal by retinoic acid. J Cell Physiol 218(1):157–166. https://doi.org/10.1002/jcp.21581
Kisseleva T, Brenner D (2021) Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 18(3):151–166. https://doi.org/10.1038/s41575-020-00372-7
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134(4):657–667. https://doi.org/10.1016/j.cell.2008.06.049
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16(11):26087–26124. https://doi.org/10.3390/ijms161125942
Liang B, Guo XL, Jin J, Ma YC, Feng ZQ (2015) Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J Gastroenterol 21(17):5271–5280. https://doi.org/10.3748/wjg.v21.i17.5271
Marquez J, Mena J, Hernandez-Unzueta I, Benedicto A, Sanz E, Arteta B, Olaso E (2016) Ocoxin (R) oral solution slows down tumor growth in an experimental model of colorectal cancer metastasis to the liver in Balb/c mice. Oncol Rep 35(3):1265–1272. https://doi.org/10.3892/or.2015.4486
Montiel-Duarte C, Ansorena E, Lopez-Zabalza MJ, Cenarruzabeitia E, Iraburu MJ (2004) Role of reactive oxygen species, glutathione and NF-kappaB in apoptosis induced by 3,4-methylenedioxymethamphetamine (“Ecstasy”) on hepatic stellate cells. Biochem Pharmacol 67(6):1025–1033. https://doi.org/10.1016/j.bcp.2003.10.020
Moro T, Shimoyama Y, Kushida M, Hong YY, Nakao S, Higashiyama R, Sugioka Y, Inoue H, Okazaki I, Inagaki Y (2008) Glycyrrhizin and its metabolite inhibit Smad3-mediated type I collagen gene transcription and suppress experimental murine liver fibrosis. Life Sci 83(15–16):531–539. https://doi.org/10.1016/j.lfs.2008.07.023
Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP (2002) Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem 277(13):11069–11076. https://doi.org/10.1074/jbc.M111490200
Nakamura M, Saito H, Ikeda M, Hokari R, Kato N, Hibi T, Miura S (2010) An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J Gastroenterol 16(2):184–192. https://doi.org/10.3748/wjg.v16.i2.184
Oakley F, Meso M, Iredale JP, Green K, Marek CJ, Zhou X, May MJ, Millward-Sadler H, Wright MC, Mann DA (2005) Inhibition of inhibitor of kappaB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis. Gastroenterology 128(1):108–120. https://doi.org/10.1053/j.gastro.2004.10.003
Pandiella-Alonso A, Diaz-Rodriguez E, Sanz E (2020) Antitumoral properties of the nutritional supplement Ocoxin oral solution: a comprehensive review. Nutrients 12(9). https://doi.org/10.3390/nu12092661
Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B, Feirt N, Mei B, Cho MS, Ramamoorthi R, Roldan G, Ng P, Lum P, Hirth-Dietrich C, Tomkinson A, Brenner DA (2004) Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology 40(5):1106–1115. https://doi.org/10.1002/hep.20425
Perez de Obanos MP, Lopez-Zabalza MJ, Arriazu E, Modol T, Prieto J, Herraiz MT, Iraburu MJ (2007) Reactive oxygen species (ROS) mediate the effects of leucine on translation regulation and type I collagen production in hepatic stellate cells. Biochim Biophys Acta 1773(11):1681–1688. https://doi.org/10.1016/j.bbamcr.2007.07.005
Ramos-Tovar E, Muriel P (2020), Molecular mechanisms that link oxidative stress, inflammation, and fibrosis in the liver. Antioxidants (Basel) 9(12) https://doi.org/10.3390/antiox9121279
Sharoni Y, Agbaria R, Amir H, Ben-Dor A, Bobilev I, Doubi N, Giat Y, Hirsh K, Izumchenko G, Khanin M, Kirilov E, Krimer R, Nahum A, Steiner M, Walfisch Y, Walfisch S, Zango G, Danilenko M, Levy J (2003) Modulation of transcriptional activity by antioxidant carotenoids. Mol Aspects Med 24(6):371–384. https://doi.org/10.1016/s0098-2997(03)00033-5
Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA (2010) Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 273(1–3):45–52. https://doi.org/10.1016/j.tox.2010.04.014
Tsuchida T, Friedman SL (2017) Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 14(7):397–411. https://doi.org/10.1038/nrgastro.2017.38
Tsukada S, Westwick JK, Ikejima K, Sato N, Rippe RA (2005) SMAD and p38 MAPK signaling pathways independently regulate alpha1(I) collagen gene expression in unstimulated and transforming growth factor-beta-stimulated hepatic stellate cells. J Biol Chem 280(11):10055–64. https://doi.org/10.1074/jbc.M409381200
Varela-Rey M, Montiel-Duarte C, Oses-Prieto JA, Lopez-Zabalza MJ, Jaffrezou JP, Rojkind M, Iraburu MJ (2002) p38 MAPK mediates the regulation of alpha1(I) procollagen mRNA levels by TNF-alpha and TGF-beta in a cell line of rat hepatic stellate cells(1). FEBS Lett 528(1–3):133–138. https://doi.org/10.1016/s0014-5793(02)03276-3
Vilar Gomez E, Gra Oramas B, Soler E, Llanio Navarro R, Ruenes Domech C (2007) Viusid, a nutritional supplement, in combination with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Liver Int 27(2):247–259. https://doi.org/10.1111/j.1478-3231.2006.01411.x
Vilar Gomez E, Rodriguez de Miranda A, Gra Oramas B, ArusSoler E, Llanio Navarro R, CalzadillaBertot L, Yasells Garcia A, del Rosario Abreu Vazquez M (2009) Clinical trial: a nutritional supplement Viusid, in combination with diet and exercise, in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther 30:999–1009. https://doi.org/10.1111/j.1365-2036.2009.04122.x
Vilar Gomez E, Sanchez Rodriguez Y, Torres Gonzalez A, Calzadilla Bertot L, Arus Soler E, Martinez Perez Y, Yasells Garcia A, Abreu Vazquez R (2011) Viusid, a nutritional supplement, increases survival and reduces disease progression in HCV-related decompensated cirrhosis: a randomised and controlled trial. BMJ Open 1(2):e000140. https://doi.org/10.1136/bmjopen-2011-000140
Vilar-Gomez E, Vuppalanchi R, Gawrieh S, Ghabril M, Saxena R, Cummings OW, Chalasani N (2020) Vitamin E improves transplant-free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology 71(2):495–509. https://doi.org/10.1002/hep.30368
Acknowledgements
We thank the technician from our group María Martínez.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by a grant from Plan de Investigación de la Universidad de Navarra (PIUNA).
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used—conceptualization, M.R.G, E.A.A., and M.J.I; methodology, M.R.G., M.P.O., and E.A.; formal analysis, M.R.G, E.A.A., and M.J.I.; resources, M.J.I.; data curation, M.R.G., E.A.A., and M.J.I.; writing—original draft preparation, M.R.G., E.A.A., and M.J.I.; writing—review and editing, M.R.G., E.A, E.A.A., and M.J.I.; supervision, E.A.A. and M.J.I.; project administration, M.J.I.; funding acquisition, M.J.I.; “all authors have read and agreed to the published the version of the manuscript.”
Corresponding author
Ethics declarations
Research involving human participants and/or animals
NA.
Informed consent
NA
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
-Ocoxin causes a decreased in the production of collagen type I in hepatic stellate cells.
-p38MAPK mediates an apoptotic effect of Ocoxin in hepatic stellate cells.
Supplementary Information
ESM 1
(PPTX 103 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruiz de Galarreta, M., Arriazu, E., Pérez de Obanos, M.P. et al. Antifibrogenic and apoptotic effects of Ocoxin in cultured rat hepatic stellate cells. J Physiol Biochem 79, 881–890 (2023). https://doi.org/10.1007/s13105-022-00878-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00878-5