Abstract
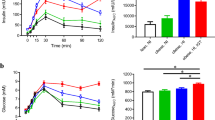
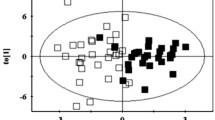
Obesity is a major contributor to the silent and progressive development of type 2 diabetes (T2D) whose prevention could be improved if individuals at risk were identified earlier. Our aim is to identify early phenotypes that precede T2D in diet-induced obese minipigs. We fed four groups of minipigs (n = 5–10) either normal-fat or high-fat high-sugar diet during 2, 4, or 6 months. Morphometric features were recorded, and metabolomics and clinical parameters were assessed on fasting plasma samples. Multivariate statistical analysis on 46 morphometrical and clinical parameters allowed to differentiate 4 distinct phenotypes: NFC (control group) and three others (HF2M, HF4M, HF6M) corresponding to the different stages of the obesity progression. Compared to NFC, we observed a rapid progression of body weight and fat mass (4-, 7-, and tenfold) in obese phenotypes. Insulin resistance (IR; 2.5-fold increase of HOMA-IR) and mild dyslipidemia (1.2- and twofold increase in total cholesterol and HDL) were already present in the HF2M and remained stable in HF4M and HF6M. Plasma metabolome revealed subtle changes of 23 metabolites among the obese groups, including a progressive switch in energy metabolism from amino acids to lipids, and a transient increase in de novo lipogenesis and TCA-related metabolites in HF2M. Low anti-oxidative capacities and anti-inflammatory response metabolites were found in the HF4M, and a perturbed hexose metabolism was observed in HF6M. Overall, we show that IR and progressively obese minipigs reveal phenotype-specific metabolomic signatures for which some of the identified metabolites could be considered as potential biomarkers of early progression to TD2.






Similar content being viewed by others
References
Boudah S, Olivier M-F, Aros-Calt S et al (2014) Annotation of the human serum metabolome by coupling three liquid chromatography methods to high-resolution mass spectrometry. J Chromatogr B 966:34–47. https://doi.org/10.1016/j.jchromb.2014.04.025
Cirera S, Taşöz E, Juul Jacobsen M et al (2020) The expression signatures in liver and adipose tissue from obese Göttingen Minipigs reveal a predisposition for healthy fat accumulation. Nutr Diabetes 10:9. https://doi.org/10.1038/s41387-020-0112-y
Curtasu MV, Knudsen KEB, Callesen H, et al (2018) Obesity development in a miniature Yucatan pig model: a multi-compartmental metabolomics study on cloned and normal pigs fed restricted or ad libitum high-energy diets. J Proteome Res acs.jproteome.8b00264. https://doi.org/10.1021/acs.jproteome.8b00264
Curtasu MV, Skou Hedemann M, Nygaard Lærke H, Bach Knudsen KE (2021) Obesity development and signs of metabolic abnormalities in young Göttingen minipigs consuming energy dense diets varying in carbohydrate quality. Nutrients 13:1560. https://doi.org/10.3390/nu13051560
Curtasu MV, Tafintseva V, Bendiks ZA et al (2020) Obesity-related metabolome and gut microbiota profiles of juvenile Göttingen minipigs—long-term intake of fructose and resistant starch. Metabolites 10:456. https://doi.org/10.3390/metabo10110456
Delgado PL (2000) Depression: the case for a monoamine deficiency. J Clin Psychiatry 61(Suppl 6):7–11
Duan Y, Sun H, Yao Y et al (2021) Perturbation of serum metabolome in relation to type 2 diabetes mellitus and urinary levels of phthalate metabolites and bisphenols. Environ Int 155:106609. https://doi.org/10.1016/j.envint.2021.106609
Dyson MC, Alloosh M, Vuchetich JP et al (2006) Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56:35–45
Faucher P, Poitou C (2016) Physiopathologie, déterminants et complications de l’obésité. Soins 61:20–25. https://doi.org/10.1016/j.soin.2016.10.003
Forouhi NG, Misra A, Mohan V, et al (2018) Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ k2234. https://doi.org/10.1136/bmj.k2234
Gastaldelli A, Gaggini M, DeFronzo RA (2017) Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio metabolism study. Diabetes 66:815–822. https://doi.org/10.2337/db16-1167
Guasch-Ferré M, Hruby A, Toledo E et al (2016) Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39:833–846. https://doi.org/10.2337/dc15-2251
Haslam DW, James WPT (2005) Obesity. The Lancet 366:1197–1209. https://doi.org/10.1016/S01406736(05)67483-1
International Diabetes Federation (2021) IDF Diabetes Atlas 2021 – 10th edition.
Jung CH, Lee WJ, Song K-H (2017) Metabolically healthy obesity: a friend or foe? Korean J Intern Med 32:611–621. https://doi.org/10.3904/kjim.2016.259
Lee H, Lee IS, Choue R (2013) Obesity, inflammation and diet. Pediatr Gastroenterol Hepatol Nutr 16:143. https://doi.org/10.5223/pghn.2013.16.3.143
Lee S, Bayless DS, Scroggins RJ et al (2015) Disconnect between adipose tissue inflammation and cardiometabolic dysfunction in Ossabaw pigs: Obese Ossabaw Pigs are Protected from Inflammation. Obesity 23:2421–2429. https://doi.org/10.1002/oby.21252
Lim EL, Hollingsworth KG, Aribisala BS et al (2011) Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54:2506–2514. https://doi.org/10.1007/s00125-011-2204-7
Liu L, Wang M, Yang X et al (2013) Fasting serum lipid and dehydroepiandrosterone sulfate as important metabolites for detecting isolated postchallenge diabetes: serum metabolomics via ultra-high-performance LC-MS. Clin Chem 59:1338–1348. https://doi.org/10.1373/clinchem.2012.200527
Low Wang CC, Lu L, Leitner JW et al (2013) Arterial insulin resistance in Yucatan micropigs with diet-induced obesity and metabolic syndrome. J Diabetes Complications 27:307–315. https://doi.org/10.1016/j.jdiacomp.2013.02.009
Ludvigsson J (2013) C-peptide in diabetes diagnosis and therapy. Front Biosci E5:214–223. https://doi.org/10.2741/E609
Patel DP, Krausz KW, Xie C et al (2017) Metabolic profiling by gas chromatography-mass spectrometry of energy metabolism in high-fat diet-fed obese mice. PLoS ONE 12:e0177953. https://doi.org/10.1371/journal.pone.0177953
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:45e–445. https://doi.org/10.1093/nar/29.9.e45
Phillips CM, Perry IJ (2013) Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab 98:E1610–E1619. https://doi.org/10.1210/jc.2013-2038
Polakof S, Rémond D, Bernalier-Donadille A et al (2018) Metabolic adaptations to HFHS overfeeding: how whole body and tissues postprandial metabolic flexibility adapt in Yucatan mini-pigs. Eur J Nutr 57:119–135. https://doi.org/10.1007/s00394-016-1302-1
Polakof S, Rémond D, David J et al (2018) Time-course changes in circulating branched-chain amino acid levels and metabolism in obese Yucatan minipig. Nutrition 50:66–73. https://doi.org/10.1016/j.nut.2017.11.004
Poupin N, Tremblay-Franco M, Amiel A et al (2019) Arterio-venous metabolomics exploration reveals major changes across liver and intestine in the obese Yucatan minipig. Sci Rep 9:12527. https://doi.org/10.1038/s41598-019-48997-2
Renner S, Blutke A, Clauss S et al (2020) Porcine models for studying complications and organ crosstalk in diabetes mellitus. Cell Tissue Res 380:341–378. https://doi.org/10.1007/s00441-019-03158-9
Sébert SP, Lecannu G, Kozlowski F, Siliart B, Bard JM, Krempf M, Champ MM (2005) Childhood obesity and insulin resistance in a Yucatan mini-piglet model: putative roles of IGF-1 and muscle PPARs in adipose tissue activity and development. Int J Obes (Lond) 29(3):324–333. https://doi.org/10.1038/sj.ijo.0802823
Shan L, Yang J, Meng S et al (2021) Urine metabolomics profiling of lumbar disc herniation and its traditional Chinese medicine subtypes in patients through gas chromatography coupled with mass spectrometry. Front Mol Biosci 8:648823. https://doi.org/10.3389/fmolb.2021.648823
Stefanovski D, Punjabi NM, Boston RC, Watanabe RM (2021) Insulin action, glucose homeostasis and free fatty acid metabolism: insights from a novel model. Front Endocrinol 12:625701. https://doi.org/10.3389/fendo.2021.625701
Steinert RE, Feinle-Bisset C, Asarian L et al (2017) Ghrelin, CCK, GLP-1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev 97:411–463. https://doi.org/10.1152/physrev.00031.2014
Strömbom U, Krotkiewski M, Blennow K et al (1996) The concentrations of monoamine metabolites and neuropeptides in the cerebrospinal fluid of obese women with different body fat distribution. Int J Obes Relat Metab Disord 20:361–368
Sun Y, Gao H-Y, Fan Z-Y et al (2020) Metabolomics signatures in type 2 diabetes: a systematic review and integrative analysis. J Clin Endocrinol Metab 105:1000–1008. https://doi.org/10.1210/clinem/dgz240
Ulaszewska MM, Weinert CH, Trimigno A et al (2019) Nutrimetabolomics: an integrative action for metabolomic analyses in human nutritional studies. Mol Nutr Food Res 63:1800384. https://doi.org/10.1002/mnfr.201800384
Vangaveti V, Baune BT, Kennedy RL (2010) Review: Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Therapeutic Adv Endocrinol 1:51–60. https://doi.org/10.1177/2042018810375656
Wan Y, Yuan J, Li J et al (2020) Overweight and underweight status are linked to specific gut microbiota and intestinal tricarboxylic acid cycle intermediates. Clin Nutr 39:3189–3198. https://doi.org/10.1016/j.clnu.2020.02.014
Wang F, Zhao Y, Niu Y et al (2012) Activated glucose-6-phosphate dehydrogenase is associated with insulin resistance by upregulating pentose and pentosidine in diet-induced obesity of rats. Horm Metab Res 44:938–942. https://doi.org/10.1055/s-0032-1323727
Wang H, Zhang H, Yao L et al (2018) Serum metabolic profiling of type 2 diabetes mellitus in Chinese adults using an untargeted GC/TOFMS. Clin Chim Acta 477:39–47. https://doi.org/10.1016/j.cca.2017.11.036
Wang Sattler R, Yu Z, Herder C et al (2012) Novel biomarkers for pre[Inline Image Removed] diabetes identified by metabolomics. Mol Syst Biol 8:615. https://doi.org/10.1038/msb.2012.43
Washburn R, Cox J, Muhlestein J et al (2019) Pilot study of novel intermittent fasting effects on metabolomic and trimethylamine N-oxide changes during 24-hour water-only fasting in the FEELGOOD trial. Nutrients 11:246. https://doi.org/10.3390/nu11020246
Wu L, Parhofer KG (2014) Diabetic dyslipidemia. Metabolism 63:1469–1479. https://doi.org/10.1016/j.metabol.2014.08.010
Xu Y, Curtasu MV, Bach Knudsen KE et al (2021) Dietary fibre and protein do not synergistically influence insulin, metabolic or inflammatory biomarkers in young obese Göttingen minipigs. Br J Nutr 125:828–840. https://doi.org/10.1017/S0007114520003141
Yamamoto T, Inokuchi T, Ka T et al (2010) relationship between plasma uridine and insulin resistance in patients with non-insulin-dependent diabetes mellitus. Nucleosides, Nucleotides Nucleic Acids 29:504–508. https://doi.org/10.1080/15257771003740986
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14:88–98. https://doi.org/10.1038/nrendo.2017.151
Acknowledgements
The authors acknowledge D. Durand and the staff of the IEN Animal Facility (B. Cohade, Y. Guerin, and J. Hermet) for technical assistance.
Funding
This study was funded by Agence Nationale de La Recherche (ANR-19-CE14-0026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures were in accordance with the guidelines formulated by the European Community for the use of experimental animals (L358-86/609/EEC, Council Directive, 1986) and approved by the Auvergne Ethical Committee (authorization 23392–2019121914101286).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• HFHS-fed minipigs showed progressive obesity stages up to almost 50% of fat mass.
• Obese minipigs developed IR, mild dyslipidemia but without overt inflammation.
• Plasma metabolomics profiles are modified along the different obesity stages.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bousahba, I., David, J., Castelli, F. et al. Despite similar clinical features metabolomics reveals distinct signatures in insulin resistant and progressively obese minipigs. J Physiol Biochem 79, 397–413 (2023). https://doi.org/10.1007/s13105-022-00940-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00940-2