Abstract
NOX5 is the last member of the NADPH oxidase (NOXs) family to be identified and presents some specific characteristics differing from the rest of the NOXs. It contains four Ca2+ binding domains at the N-terminus and its activity is regulated by the intracellular concentration of Ca2+. NOX5 generates superoxide (O2•−) using NADPH as a substrate, and it modulates functions related to processes in which reactive oxygen species (ROS) are involved. Those functions appear to be detrimental or beneficial depending on the level of ROS produced. For example, the increase in NOX5 activity is related to the development of various oxidative stress-related pathologies such as cancer, cardiovascular, and renal diseases. In this context, pancreatic expression of NOX5 can negatively alter insulin action in high-fat diet-fed transgenic mice. This is consistent with the idea that the expression of NOX5 tends to increase in response to a stimulus or a stressful situation, generally causing a worsening of the pathology. On the other hand, it has also been suggested that it might have a positive role in preparing the body for metabolic stress, for example, by inducing a protective adipose tissue adaptation to the excess of nutrients supplied by a high-fat diet. In this line, its endothelial overexpression can delay lipid accumulation and insulin resistance development in obese transgenic mice by inducing the secretion of IL-6 followed by the expression of thermogenic and lipolytic genes. However, as NOX5 gene is not present in rodents and human NOX5 protein has not been crystallized, its function is still poorly characterized and further extensive research is required.
Similar content being viewed by others
NADPH oxidases
The NADPH oxidase (NOXs) family is characterized for being membrane proteins that use NADPH as an electron donor. The first enzyme belonging to this family of oxidases was identified in 1986. It was located in the membrane of phagocytic cells and it was found to be a protein capable of generating H2O2 using NADPH as a substrate. This enzyme was named NOX2/gp91PHOX. Since then, six more isoforms have been identified, constituting the family of NADPH oxidases (Fig. 1), whose 7 members are called NOX 1–5 and dual oxidases DUOX 1–2 [15, 17, 94]. As explained below, the DUOX family constitutes a different subfamily due to their extended primary sequence, the type of ROS formed, their maturation process, and their functional regulation [29]. The NOX members share the same catalytic core: a six-transmembrane helical domain (TM) with a C-terminal cytosolic dehydrogenase domain (DH). The DH domain includes the binding sites for FAD (flavin adenine dinucleotide) and NADPH, whereas TM binds two hemes [15, 73].
Schematic representation of the structure of the different NOXs. A Structure of isoforms 1–3. B Structure of isoform 4. C Structure of isoform 5. D Structure of DUOX1-2 isoforms. The transmembrane domains (blue), the subunits necessary for the activation of each enzyme (brown), the stabilizer subunits (orange), and maturation factors in DUOX (red) and the FAD/NADH binding domains (purple) are indicated in each figure. Image adapted from Buvelot et al. [17]
NOX2 is the most studied member of those oxidases. It can be found in cardiomyocytes, myocytes, hepatocytes, and endothelial cells. Its gp91phox subunit, also cataloged as NOX2, represents the catalytic center. The p22phox, p47phox, and p67phox subunits form the stabilizing, organizing, and activating subunits, respectively [44, 46, 49, 55, 92]. The mechanism by which this enzyme and the rest of NOXs generates O2•− is the following: NADPH binds to FAD and transfers two electrons to it. As a result, FAD is reduced to FADH2 and transfers an electron to the heme group located on the gp91phox subunit. Consecutively, the latter will reduce the O2 molecule to produce O2•−. FADH• can repeat the process faster generating another molecule of O2•− [28, 46].
The NOX1 isoform has 60% sequence identity to NOX2. It is expressed in various tissues such as the epithelium of the colon, uterus, prostate, and muscle and in endothelial cells. Like NOX2, NOX1 acts as a catalytic center for the generation of O2•−; p22phox is the stabilizing subunit; NOXO1 is the organizer; and NOXO1A is the activator [2, 10, 99]. In the case of NOX3, its sequence identity to NOX2 is 56%. Like NOX1, this enzyme complex is composed of NOX3, p22phox, NOXO1, and NOXO1A. It is expressed in tissues such as the inner ear and its main characteristic is that it can be activated without the need for an external stimulus [11, 104].
Interestingly, NOX4 protein is not as similar to NOX2 as the previous isoforms. Its distribution is especially abundant in the kidney, but it is also expressed in endothelium and muscle. The enzymatic complex of this protein is formed only by two proteins: NOX4, which is the catalytic center, and p22phox. Furthermore, it does not require the binding of cytosolic subunits to carry out its activity. NOX4 presents an activity similar to that of superoxide dismutase (SOD) so that the superoxide that is generated is rapidly converted to H2O2 [41, 53, 83].
Lastly, there are the DUOX 1 and 2. These isoforms present a high level of expression in the thyroid gland and they are known as thyroid oxidases, although they have also been identified in the gastrointestinal tract and prostate. The main difference between DUOXs and the rest of NOXs is that they present an additional intracellular region containing two EF-hands motifs preceded by a seventh transmembrane fragment ended by an extracellular peroxidase-homology domain (PHD) in the N-terminal region, and require the presence of maturation factors (DUOXA1/2) for their activity. Like NOX4, DUOX1 and 2 generate H2O2, whose production is dependent on calcium and phosphorylation [4, 17, 29, 30, 45, 68, 105].
NOX5
The last member of the NOX family to be identified was NOX5. In 2001, two independent research groups first detected the mRNA of a protein with high homology to NOX1 and 2. This mRNA encoded the NOX5 protein, and was detected in spleen, kidney, and testis samples. Despite the high degree of homology, NOX5 presented a great structural difference compared to the rest of NOXs, the presence of calcium-binding domains (Ca2+) at the N-terminus [12, 26]. From the research carried out by Cheng et al., it was determined that the human NOX5 protein constituted its own phylogenetic branch, quite different from the rest of the NOXs. This suggests that NOX5 perhaps represents the most similar protein to the ancestral NOX [26]. Due to several evolutionary studies, it has been observed that various plants, animals, and some fungi incorporate enzymes similar to NOX5, which suggests that the appearance of the first NOX5 occurred before the evolutionary separation of the kingdoms. These analyses have also served to find out that this protein has disappeared or duplicated itself in some organisms. For example, up to 10 NOX5-like genes have been detected in plants. In contrast, in organisms such as rodents its existence has not been detected [14, 63].
Based on this information, some experts refer to this oxidase as “the enigmatic NOX” [103]. The reasons that justify this name are as follows:
-
1.
NOX5 gene is not present in rodents.
-
2.
It does not require the union of other subunits to be active.
-
3.
Its structure incorporates four Ca2+ binding domains at the N-terminus.
-
4.
Its activity is regulated by the intracellular concentration of Ca2+.
-
5.
The activation of this oxidase requires protein conformational changes.
-
6.
NOX5 is not N-glycosylated, in contrast to other NOXs.
NOX5 gene
In humans, the NOX5 gene is located on the long arm of chromosome 15 and is composed of 18 exons. Through alternative splicing of exons, six variants have been identified: α, β, δ, γ, ε (truncated variant), and ζ. Two gene transcription start sites have been identified by studies using the 5′ RACE technique. One of them is for the β and δ variants (exon 4) and another is for the α and γ variants (exon 3). In the case of the ε isoform, its start is located in exon 6. The rest of the exons are common to all isoforms [14, 35, 97, 103].
As mentioned above, NOX5 mRNA was initially identified in testis, spleen, and kidney samples [12, 26]. Over the years, the presence of this enzyme in other tissues and cell types has been demonstrated. This is the case of endothelial cells, vascular smooth muscle cells, and cardiomyocytes of the cardiovascular system [74, 80, 108]. Other tissues where this protein is expressed are bone marrow, uterus, stomach, skeletal muscle, and in the placenta [103]. Previous work carried out in our research group also demonstrated its expression in hepatic stellate cells [6]. Regarding the isoforms, their expression depends on the cell type analyzed. The β variant is usually the most frequent and has been detected in lung smooth muscle or podocytes. For its part, the ε variant, which was thought to be practically inactive, causes the increase in reactive oxygen species (ROS) characteristic of Barrett’s carcinoma [86, 98].
The promoter region of the gene presents alternative binding sites for different transcription factors as follows: AP-1, C/EBP, NF-κB, and STAT [75, 86]. Moreover, NOX5 gene expression can be induced by using different agonists. Angiotensin II represents an example of an agonist whose binding to its receptor in vascular smooth muscle and endothelial cells can produce an increase in the expression of NOX5 both at the mRNA and protein levels [80]. In the same work, the existence of another agonist, endothelin 1, was also confirmed. NOX5 is a protein capable of inducing various cellular changes when it binds to the endothelin receptor. One of these changes is the increase in ROS levels. Other molecules that have been described as NOX5 inducers are platelet-derived growth factor (PDGF), tumor necrosis factor-alpha (TNFα), or leptin [56, 74, 86].
Concerning the epigenetic regulation of the gene, some microRNAs (miRNAs), a class of non-coding RNAs that play important roles in regulating gene expression, have been postulated to modulate NOX5 expression. Thus, miR-15a-3p, present in circulating exosomes from diabetic patients, appears to reduce NOX5 expression within human endothelial cells [107]. On the other hand, in an in vitro model of diabetic nephropathy, miR-485 suppresses inflammation and proliferation of human mesangial cells by suppressing the expression of NOX5 [106]. Finally, the in silico analysis of miR-4321 and miR-4270, miRNAs that are upregulated in the serum of sepsis-induced acute kidney injury patients, predicts that they are able to decrease NOX5 expression [40].
Structure and localization of NOX5
The human NOX5 protein has not yet been crystallized. However, in 2017, the structure of the ortholog of the cyanobacterium Cylindrosperum stagnale was determined. Due to this work, it has been possible to carry out experiments that allow studying the mechanism of O2•− production derived from FAD domains [73].
From the crystallized structure and through comparative studies with other NOXs, it has been concluded that the main components for electron transport are conserved in the NOX5 protein (Fig. 2). These are the six transmembrane domains with two histidine-linked Fe groups that link α-helices 3 and 5, the FAD-binding domain, and the NADPH-binding motif located at the C-terminus [12]. Along with these conserved elements, NOX5 includes other characteristics that influence its biochemical properties. The major difference over other enzymes of the same family is that, as mentioned above, the N-terminal end of NOX5 contains four EF-hands motifs, which allow the binding of Ca2+ ions. It also includes a polybasic domain (PBR-N), which is present at the carboxyl end (PBR-C). It is a region rich in residues susceptible to being phosphorylated (S and T) and with a characteristic calmodulin-binding region [13, 35].
Schematic representation of the structure of NOX5. The protein contains six transmembrane domains (dark blue), with the N- and C-terminus disposed toward the cytosol. At the amino end, the EF-hand motifs are located, capable of binding Ca2+ ions (golden). The FAD domain (yellow) and the NADPH binding motif (purple), responsible for the production of O2.•−, and the polybasic domain (PBR-C) (pale blue) are located at the carboxyl end. Image adapted from Touyz et al. [103]
In the case of the NOX5 variants, with the exception of the δ isoform, there are no major differences in their structure (Fig. 3). All NOX5 variants present six transmembrane domains and the same characteristics of the C-terminal region. However, at the amino terminus, there are slight divergences among variants. The α and β isoforms are the most active and they do not present insertions among the EF-hands motifs. In contrast, in the case of γ and δ, there is an insertion between these motifs that seems to affect the activity of the enzyme. The ε isoform, which does not seem to present much activity either, is characterized by the absence of EF-hands motifs. Finally, the ζ isoform is quite similar to the α but differs in that it hardly shows any activity [86].
Schematic representation of the structure of the six NOX5 variants. The transmembrane domains and the C-terminus are identical for all isoforms. The main differences are present at the amino end. Image adapted from Fulton et al. [35]
Finally, in contrast to the rest of NOXs, NOX5 is mainly located in intracellular membranes such as the endoplasmic reticulum and the perinuclear area. Its presence in the plasma membrane appears to be limited to the release of ROS into the extracellular space [3, 62]. The reason why NOX5 localizes to the reticulum is not entirely clear but this might be related to the fact that it is an organelle rich in intracellular Ca2+, thus establishing a regulation of NOX5 via the endoplasmic reticulum [81]. The transition of NOX5 from the reticulum to the plasma membrane seems to be mediated by the carboxy-terminal PBR domain, as it is capable of binding the membrane phospholipid phosphatidylinositol 4,5-bisphosphate [62].
Regulation of NOX5 activity
The molecular mechanism by which NOX5 generates O2•− is the same as the one described above for NOX2. Briefly, NADPH donates electrons to the FAD domain, which binds to the heme group of the transmembrane regions. This entails the reduction of the O2 molecule to generate O2•−. However, the regulation of NOX5 activity presents relevant differences compared with other oxidases of the family. Although NOX5 can bind to the p22phox subunit, it has been described that such interaction is not required for NOX5 activity [91]. In fact, as observed at the time of its discovery, the activity of the enzyme depends mainly on its binding to Ca2+ [12, 26].
Calcium-dependent allosteric regulation
As previously mentioned, the regulatory domain (called NOX5-EF) present in the N-terminal end of NOX5, includes four EF-hand domains that allow Ca2+ binding. These motifs act two by two, with those closer to the end having the lowest affinity for Ca2+. The binding of Ca2+ to these motifs induces a conformational change in the structure of NOX5 characterized by the interaction of some hydrophobic residues of the amino end with the C-terminal catalytic domain activating the enzyme. The C-terminus incorporates an autoinhibitory domain called the EF-hand binding domain. Interaction of the Ca2+-bound N-terminus with this region removes the inhibition, facilitating the activation of the enzyme [13, 101]. The production of ROS derived from NOX5 requires high concentrations of intracellular Ca2+. Nevertheless, since the enzyme is capable of producing ROS in situations where Ca2+ concentrations are low, there must be other mechanisms that regulate NOX5 activity.
Regulation by covalent modification (phosphorylation) of NOX5
In 2007, Jagnandan D. et al. [54] demonstrated another form of regulation of NOX5 activity, independent of Ca2+. These studies found that the protein kinase C (PKC) activator compound phorbol 12-myristate 13-acetate (PMA) was able to activate NOX5 without an increase in intracellular Ca2+ levels. In addition, a synergy between PMA and Ca2+ was observed. That is, the presence of PMA caused the enzyme to be even more sensitive to the action of Ca2+. At the C-terminal end of the protein, there is a series of serine and threonine residues (T512 and S516) that can be phosphorylated by PKC. Mutation of these residues to alanine reduced NOX5 activation in the presence of PMA [54]. Within the PKC family, phosphorylation was found to be dependent on the α isoform as its silencing reduced the ability of PMA to activate O2•− production [22]. Likewise, it seems that the kinases regulated by extracellular signals (ERK1/2) would also regulate the activity of NOX5 [84].
Other types of regulation
NOX5 activity can also be regulated by other types of post-translational modifications. These changes can occur through oxidation, nitrosylation, sumoylation, and palmitoylation. The oxidation of the enzyme seems to cause a reduction in its activity since it reduces the Ca2+ binding capacity of the protein. Nitrosylation, together with sumoylation, also reduce its activity. Instead, palmitoylation appears to regulate the cellular localization of the enzyme [103].
Taking into account the role played by PKC in the regulation of oxidase activity, the possibility that other proteins could interact with NOX5, modifying its activity, has been evaluated. In this sense, the first protein described was calmodulin, for which NOX5 displays a binding domain at the C-terminal end and allows the oxidase to be active without the need for high Ca2+ levels. In turn, it has been reported that certain calmodulin inhibitors such as KN-93 are also capable of inhibiting the activity of NOX5 [85].
NOX5 also interacts with the chaperone Hsp90. This interaction results critical for the oxidase activity, since its inhibition compromises the ability of NOX5 to generate O2•−. Furthermore, inhibition of Hsp90 leads to the binding of another chaperone, Hsp70, which induces the degradation of NOX5 in the proteasome [72]. Finally, another protein that exhibits direct binding to NOX5 is caveolin 1 (Cav1). Previously, the existence of a direct union between Cav1 and NOX2 had already been described, a fact that facilitated the activation of this member of the family [71]. In the case of NOX5, in 2014, an article was published for the first time linking both proteins and reflecting the opposite of what was observed with NOX2. Cav1 negatively regulated the activity of not only NOX5, but also NOX1-3 [21]. In fact, in 2020, the presence of NOX5 and NOX1 in the caveolae of the plasma membrane in smooth muscle cells was established and the negative regulatory role that Cav1 plays on the activity of both enzymes was confirmed [5].
Role of NOX5 in physiology and pathophysiology
NOX5 in physiology
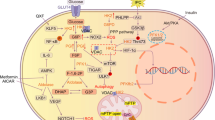
The presence of NOX5 in different cell types and the variability in its expression make it ambiguous whether its expression promotes positive or negative effects on the tissues in which it is expressed. Its basal activity could be related to physiological effects mediated by oxidative signaling, whereas its overexpression or activation tends to cause oxidative stress-induced physiopathological damage (Fig. 4).
Cellular differentiation
The first studies dedicated to this protein determined that NOX5 is expressed in human monocytes and macrophages and that it is capable of regulating the differentiation of monocytes to dendritic cells [78]. However, these studies were carried out with leukemia cell lines, so the physiological effects of NOX5 on neutrophils are not entirely clear. Regarding the involvement of NOX5 in cellular differentiation, it has been confirmed that NOX5 is required for the proper differentiation of human oligodendrocytes [1].
Cardiovascular function
NOX5 is also expressed in other non-immune cell types and appears to modulate functions related to processes in which ROS are involved. In this sense, physiological levels of ROS are essential for normal vascular functions, including endothelial homeostasis and smooth muscle cell contraction [25]. The NOX family represents the principal enzymatic source of ROS in the vasculature [77]. Additionally, among the NOX isoforms present in human vessels, NOX5 seems to be the major ROS-generating oxidase [60, 102]. In this system, the presence of NOX5 has been confirmed at the vascular wall in endothelial cells, vascular smooth muscle cells (VSMC), and interstitial fibroblasts, as well as in blood cells themselves, such as circulating monocytes and macrophages [60]. Considering all these factors, it has been described the involvement of NOX5 activity in the physiological regulation of vascular tone [81] and angiogenesis [89]. Moreover, in relation to cardiac muscle, as previously mentioned, NOX5 is slightly expressed at the endothelium of intramyocardial blood vessels and in cardiomyocytes [48, 109], and both Ca2+ and ROS-mediated signaling are responsible for controlling cardiomyocyte contraction. In this context, NOX5 has been shown to have a key role in the regulation of muscle cell contraction, cell proliferation, and capillary generation [81, 89]. However, the expression of NOX5 in necrotic and non-necrotic as well as hypertrophied areas of the myocardium makes its role in the cardiovascular system difficult to interpret [48].
Sperm functionality
Furthermore, ROS (and more precisely O2•−) are involved in sperm functionality under physiological condition, and they are required for processes such as hyperactivation, acrosome reaction, and sperm-oocyte fusion [65]. It had been previously described that the mRNA of NOX5 is highly expressed in human testis [12], and the expression of the oxidase was identified in human [82], canine [96], and equine [95] spermatozoa. Taking all these data into account, Ghanbari et al. demonstrated that NOX5 participates in the regulation of human progesterone-activated sperm motility and viability [42]. Moreover, some studies developed in ram demonstrated that it is also involved in sperm capacitation [79].
NOX5 in pathophysiology
On the other hand, the increase in ROS levels in the body is associated with the development of oxidative stress. For this reason, the increase in NOX5 activity is related to the progression of various pathologies such as vascular and renal diseases and cancer.
Cardiovascular diseases
In the case of vascular diseases, as previously mentioned, the presence and activity of the oxidase in different cell types of this system makes the circulatory system more sensitive to alterations of NOX5. Thus, human and animal studies suggest that NOX5 might promote endothelial dysfunction and inflammation in the vasculature [77]. Inflammation and oxidative stress are currently regarded as important components of atherosclerosis pathogenesis, a process in which lipid-containing plaques are formed in the blood vessel wall that can obstruct the vessel lumen or provoke erosion or rupture, inducing further thrombotic events. The implication of the NADPH family members and their role in atherosclerosis has been previously reviewed in Poznyak et al. [90]. Regarding the precise implication of NOX5 in this process, it has been described that the mRNA and protein levels of NOX5 as well as the calcium-dependent NADPH oxidase activity are significantly increased in the atherosclerotic coronary arteries from patients with coronary artery disease as compared to nonatherosclerotic vessels [47]. However, in a recent work employing a humanized Nox5 knock-in mice that expressed NOX5 in endothelial cells to test the pro-atherogenic hypothesis, Ho et al. [50] found that the expression of the oxidase per se was insufficient to induce aortic atherosclerotic lesions, even in aged mice and exposed to a high cholesterol atherogenic diet. Moreover, in the same work, the authors demonstrated that the endothelial expression of NOX5 did not aggravate aortic atherosclerosis in the atherosclerosis-prone ApoE−/− mice with and without induction of diabetes [50]. Another process related to vascular diseases in which NOX5 appears to be involved is in vascular calcification, the formation of calcium phosphate crystals in the vessel wall that promotes vascular stiffness. In vitro studies employing hVSMCs demonstrated that the oxidase would be mediating the phenotypic switching between the normal “contractile VSMCs” to a dedifferentiated “synthetic VSMCs.” In addition, the work presents that in these synthetic VSMCs, Ca2+-NOX5-induced ROS would induce an increase in the release of extracellular vesicle (EV) as well as a decrease in their uptake, causing their extracellular accumulation and increasing calcification [36]. In the case of cardiovascular diseases, the expression of the oxidase seems to be increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction [48] and in the cardiomyocytes of failing human hearts [109]. Due to the regulatory role it exerts on cardiac tissue, NOX5 also seems to be involved in the development of heart attacks, hypertension, and cardiac hypertrophy [52, 64, 109]. Our group has also observed that NOX5-derived ROS may modulate the COX-2/PGE2 axis in endothelial cells, which might play a relevant role in the pathophysiology of heart infarction [76].
Neurological diseases
In the case of the brain, the works from the group of Harald Schmidt associate the expression of human NOX5 in endothelial and hematopoietic cells in a knock-in mouse model and in vitro organotypic hippocampal cultures, with the presence of hypertension and the risk of stroke [20, 64]. The authors show that NOX5-dependent ROS formation compromises the integrity of the blood–brain barrier, increasing infarct size and aggravating neurological function after cerebral ischemia and reperfusion injury.
Diabetic nephropathy
Likewise, it has been observed that NOX5 is expressed in renal tubule cells and that in conditions of diabetic nephropathy, its overexpression affects the correct functioning of the renal system. Specifically, NOX5 overexpression produces an increase in inflammatory cytokines that result in the appearance of kidney damage [52, 60]. In this sense, a recent paper of Jha et al. suggests that NOX 5 could be playing a much more prominent role than NOX4 in this disease [59].
Cancer
Elevated NOX5 levels have been also reported in various types of tumors and cancer cell lines (lymphoma, Barrett’s esophageal adenocarcinoma, gastric, melanoma, colon, breast, and prostate), where an increase in ROS levels correlates with increased cell proliferation, DNA damaged, angiogenesis, and reduced apoptosis [7, 9, 33, 37, 69, 93].
The activity of the oxidase has been associated with different roles in cancer progression. At the cellular level, in vitro studies have confirmed that NOX5 promotes proliferation and survival and reduced apoptosis in prostate carcinoma cancer cells, breast cancer cells, lymphoma, Barrett esophageal adenocarcinoma cells, and melanoma [7, 19, 33, 34, 51]. Using human colon and breast cancer cell lines in which the expression of NOX5 was depleted employing specific siRNAs, some works demonstrated the direct involvement of NOX5 in the migration or cell motility of those transformed cell lines [8, 33]. In addition, the activity of NOX5 is also responsible for the increased tumor cell invasion of human prostate, colon, and breast tumor cell lines [8, 33, 66]. Moreover, NOX5 could be acting as a driving oncoprotein through the regulation of the expression of several cytokines to provide the conditions that facilitates tumor malignant progression [24].
On the other hand, the clinical relevance of the activity of NOX 5 in oncology patients presenting different types of cancers has also been described. Hence, NOX5 expression was found to be increased at the invasive front of prostate cancer tumors, increasing the ROS levels in the area. The ROS produced, could potentially contribute to the expression of an active form of HIF1, that in turn, can increase the expression of the metalloproteinase 14, which was found to be also increased at the invasive front of prostate cancer tumors. NOX5 expression could be thus contributing to conferring an invasive advantage to tumor cells [66]. In the case of lymphomas, the work of Gonçalves et al. [43] demonstrated that NOX5 upregulation was associated with the acquisition of an aggressive phenotype. NOX5 expression was increased in esophageal squamous cell carcinoma (ESCC) tumors, and this elevated NOX5 was correlated to malignancy of (ESCC) tumors and poor prognosis, as a strong expression of the oxidase positively associated with advanced-stage, higher grade tumor status and higher grade lymph node status. Furthermore, those patients presenting a higher expression of NOX5 showed a shorter overall survival time. NOX5 induced the malignant progression of ESCC by activating the proto-oncogenic protein tyrosine kinase Src, particularly under hypoxic conditions [23]. NOX5 upregulation was also associated as a poor prognostic factor and worse prognosis in colon cancer patients, as the 5-year progression-free survival rate of NOX5-positive patients was significantly worse than that observed in NOX5-negative patients. Those NOX5-positive patients presented a higher local recurrence rate than that observed in NOX-5-negative patients. Besides, NOX-5 positive expression was significantly correlated with poorer differentiated histology [9].
Roles of NOX5 to be determined
Recent studies have also suggested the involvement of NOX5 in the development of other different process in which the precise function and physiological or pathophysiological consequences of the presence and activity of the oxidase are not yet fully characterized [103]. In this context, the implication of other NADPH oxidases and oxidative stress in pulmonary hypertension or neurodegenerative disorders and neuroinflammation has been previously described, though the precise role of NOX5 in those events remains elusive [87, 100]. Related to neurological disorders, our group has recently described that the endothelial expression of NOX5 in a knock-in mouse model alters the integrity of the blood–brain barrier causing loss of memory in aged animals [27]. Other work from our group also described for the first time expression and functional relevance of NOX5 in the human cell line of hepatic stellate cells (HSC) LX-2 [6]. This cell line, when activated, presents a proliferative and myofibroblastic phenotype with increased production of collagen type I and other extracellular matrix proteins responsible for the development of liver fibrosis. The work of Andueza et al. [6] confirmed that NOX5 activity contributes to the proliferation of the cell line as well as the increased production of collagen type I, pointing to a possible implication of the oxidase in the initiation and progression of liver fibrosis, in line with the role previously reported played by NOX1,NOX2, and NOX4 [70].
Metabolic diseases
In relation to metabolic diseases, several works have analyzed the effect of NOX5 in diabetes. It has been described that high concentrations of glucose can increase NOX5 expression and activity [16, 22]. The activity of the oxidase tends to demonstrate a detrimental effect in this condition, mainly due to the induction of vascular complications such as diabetic nephropathy [57], but it is also related to other complications related to the disease as the presence of foot ulcers [107], vascular retinopathy [31], or the formation of abdominal aortic aneurysms [50]. In this sense, the kidneys are probably the organs in which the effect of the activity of NOX5 has been better characterized in this situation. In patients with diabetes, there is an increased expression of renal NOX5 associated with enhanced ROS formation and the upregulation of ROS-sensitive pathways as early growth response 1 (EGR-1), protein kinase C-α (PKC-α), and thioredoxin-interacting protein (TXNIP). Moreover, in animal models of diabetic kidney diseases, the overexpression of NOX5 enhanced kidney damage by increasing albuminuria, inflammation, and renal fibrosis due to the increase of ROS and the activation of the previously mentioned ROS-sensitive pathways [58]. A recent work suggested that pancreatic (β‐cell‐specific) doxycycline‐inducible expression of NOX5 in RIP/rtTA/NOX5 transgenic mice can negatively alter insulin action in high-fat diet-fed mice [16]. All these data are consistent with the idea that the expression of NOX5 tends to increase in several diseases, generally causing a worsening of the pathology [57].
However, other studies have suggested that the activity of NOX5 could present beneficial effects in other diseases, leading to an improvement in certain conditions such as cardiac remodeling or the differentiation of monocytes into dendritic cells [48, 78]. For example, disruption of the NOX5 gene employing CRISPR/Cas9 aggravates atherosclerosis in rabbits that were administered an atherogenic diet based on a high-fat cholesterol-rich (0.5% w/w) diet to induce plaque formation, suggesting a protective role for the oxidase against atherosclerosis in this model [88]. Some works from our group demonstrated that knock-in transgenic mice expressing endothelial NOX5, presented lower body weight gain and less mesenteric and epididymal fat mass compared to control mice. Those endothelial NOX5-expressing transgenic mice also showed significantly lower glycaemia and improved insulin-induced glucose uptake, which was accompanied by increased expression of Glut4 and Cav1 in the adipose tissue of these animals [38]. Likewise, 3T3-L1 adipocytes treated with conditioned media from NOX5-expressing endothelial cells previously incubated with high glucose and palmitic acid, presented lower lipid accumulation and higher glucose uptake. In this context, another study from our group showed that, in high-fat diet-fed endothelial NOX-5-expressing transgenic mice, endothelial NOX5 activity promotes thermogenesis and lipolysis in the mesenteric and epididymal fat through phosphorylation of STAT3 and AMPK, respectively [39]. Those effects were mediated by activating interleukin-6 (IL-6) production. Moreover, 3T3-L1 adipocytes treated with conditioned media of endothelial NOX5-expressing cells previously incubated with high glucose and palmitic acid also presented higher expression of thermogenic and lipolytic genes. All these examples illustrate the need for additional studies in order to address other potentially beneficial effects that NOX5 activity could be mediating in other diseases.
As mentioned above, in the recent years, the development of transgenic models of the expression of NOX5 in mice, has enabled to demonstrate the involvement of the oxidase in several new functions. In some of those new roles, as in the field of metabolic diseases, liver fibrosis, pulmonary hypertension, neurodegenerative disorders, or neuroinflammation, the precise effect of the activity of NOX5 has to be better characterized, since it is not still clear whether its activity is being part of the damage, or an attempt of the body to activate compensatory mechanisms to solve an insult [6, 38, 39, 87, 100]. In this sense, it would be desirable to study how the expression and activity of NOX5 are modulating the endothelial function in processes that result critical in vascular structures involved in natural barriers in the organism like the blood–brain barrier, renal glomerulus or the alveoli of the lungs. Besides, further studies should be performed in order to obtain specific inhibitors for this oxidase. In this regard, it is the first and only crystallized NADPH oxidase and there are relevant studies suggesting that NOX5 could be a good therapeutic target in vascular diseases as diabetic nephropathy [59, 67, 87], cerebral ischemic injury [18], acute myocardial infarction or stroke [77], as well as in some types of cancer [9] and a potential target of cancer cell sensitivity to chemotherapies, such as cisplatin [32, 61]. Lastly, future works would be needed to explore the epigenetic regulation of the NOX5 gene and the posttranslational regulation of the protein which are still unknown so far, and the interaction with other proteins that affects its activity.
Conclusion
In summary, in humans, NOX5 modulates functions related to processes in which ROS participate. Although in some cases, NOX5 has been associated with the development and worsening of several pathologies and metabolic complications, such as in cardiac ischemia or pancreatic beta-cells, in other circumstances it may play a beneficial role in the condition of metabolic stress by adapting adipocyte metabolism. For example, it seems to induce a protective adipose tissue adaptation to the excess of nutrients caused by a high-fat diet in order to avoid, or at least delay, lipid accumulation and insulin resistance development. This may be mediated by an activation of the secretion of IL-6. Nonetheless, further research is needed in order to totally unveil the role of this protein in human health and disease.
References
Accetta R, Damiano S, Morano A, Mondola P, Paterno R, Avvedimento E, Santillo M (2016) Reactive oxygen species derived from NOX3 and NOX5 drive differentiation of human oligodendrocytes. Front Cell Neurosci 10:146. https://doi.org/10.3389/fncel.2016.00146
Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, Wakisaka M, Kawahara T, Rokutan K, Ibayashi S, Iida M (2005) NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke 36:1040–1046. https://doi.org/10.1161/01.STR.0000163111.05825.0b
Ahmarani L, Avedanian L, Al-Khoury J, Perreault C, Jacques D, Bkaily G (2013) Whole-cell and nuclear NADPH oxidases levels and distribution in human endocardial endothelial, vascular smooth muscle, and vascular endothelial cells. Can J Physiol Pharmacol 91:71–79. https://doi.org/10.1139/cjpp-2012-0265
Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C (2005) Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem 280:30046–30054. https://doi.org/10.1074/jbc.M500516200
Anagnostopoulou A, Camargo LL, Rodrigues D, Montezano AC, Touyz RM (2020) Importance of cholesterol-rich microdomains in the regulation of Nox isoforms and redox signaling in human vascular smooth muscle cells. Sci Rep 10:17818. https://doi.org/10.1038/s41598-020-73751-4
Andueza A, Garde N, Garcia-Garzon A, Ansorena E, Lopez-Zabalza MJ, Iraburu MJ, Zalba G, Martinez-Irujo JJ (2018) NADPH oxidase 5 promotes proliferation and fibrosis in human hepatic stellate cells. Free Radic Biol Med 126:15–26. https://doi.org/10.1016/j.freeradbiomed.2018.07.013
Antony S, Jiang G, Wu Y, Meitzler JL, Makhlouf HR, Haines DC, Butcher D, Hoon DS, Ji J, Zhang Y, Juhasz A, Lu J, Liu H, Dahan I, Konate M, Roy KK, Doroshow JH (2017) NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates normoxic HIF-1alpha and p27(Kip1) expression in malignant melanoma and other human tumors. Mol Carcinog 56:2643–2662. https://doi.org/10.1002/mc.22708
Ashizawa N, Shimizu H, Shoda K, Furuya S, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida H, Sudo M, Inoue S, Kono H, Katsurahara K, Shiozaki A, Ichikawa D (2021) NADPH oxidase 5 has a crucial role in cellular motility of colon cancer cells. Int J Oncol 59:63. https://doi.org/10.3892/ijo.2021.5243
Ashizawa N, Shimizu H, Sudo M, Furuya S, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida H, Inoue S, Kono H, Ichikawa D (2019) Clinical significance of NADPH oxidase 5 in human colon cancer. Anticancer Res 39:4405–4410. https://doi.org/10.21873/anticanres.13611
Banfi B, Clark RA, Steger K, Krause KH (2003) Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278:3510–3513. https://doi.org/10.1074/jbc.C200613200
Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH (2004) NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279:46065–46072. https://doi.org/10.1074/jbc.M403046200
Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH (2001) A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276:37594–37601. https://doi.org/10.1074/jbc.M103034200
Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA (2004) Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5). J Biol Chem 279:18583–18591. https://doi.org/10.1074/jbc.M310268200
Bedard K, Jaquet V, Krause KH (2012) NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52:725–734. https://doi.org/10.1016/j.freeradbiomed.2011.11.023
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313. https://doi.org/10.1152/physrev.00044.2005
Bouzakri K, Veyrat-Durebex C, Holterman C, Arous C, Barbieux C, Bosco D, Altirriba J, Alibashe M, Tournier BB, Gunton JE, Mouche S, Bietiger W, Forterre A, Berney T, Pinget M, Christofori G, Kennedy C, Szanto I (2020) Beta-cell-specific expression of nicotinamide adenine dinucleotide phosphate oxidase 5 aggravates high-fat diet-induced impairment of islet insulin secretion in mice. Antioxid Redox Signal 32:618–635. https://doi.org/10.1089/ars.2018.7579
Buvelot H, Jaquet V, Krause KH (2019) Mammalian NADPH oxidases. Methods Mol Biol 1982:17–36. https://doi.org/10.1007/978-1-4939-9424-3_2
do Carmo LS, Berk BC, Harrison DG (2019) NOX5 as a therapeutic target in cerebral ischemic injury. J Clin Investig 129:1530–1532. https://doi.org/10.1172/JCI127682
Carnesecchi S, Rougemont A-L, Doroshow JH, Nagy M, Mouche S, Gumy-Pause F, Szanto I (2015) The NADPH oxidase NOX5 protects against apoptosis in ALK-positive anaplastic large-cell lymphoma cell lines. Free Radic Biol Med 84:22–29. https://doi.org/10.1016/j.freeradbiomed.2015.02.027
Casas AI, Kleikers PW, Geuss E, Langhauser F, Adler T, Busch DH, Gailus-Durner V, de Angelis MH, Egea J, Lopez MG, Kleinschnitz C, Schmidt HH (2019) Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J Clin Invest 129:1772–1778. https://doi.org/10.1172/JCI124283
Chen F, Barman S, Yu Y, Haigh S, Wang Y, Black SM, Rafikov R, Dou H, Bagi Z, Han W, Su Y, Fulton DJ (2014) Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med 73:201–213. https://doi.org/10.1016/j.freeradbiomed.2014.04.029
Chen F, Yu Y, Haigh S, Johnson J, Lucas R, Stepp DW, Fulton DJ (2014) Regulation of NADPH oxidase 5 by protein kinase C isoforms. PLoS One 9:e88405. https://doi.org/10.1371/journal.pone.0088405
Chen J, Wang Y, Zhang W, Zhao D, Zhang L, Fan J, Li J, Zhan Q (2020) Membranous NOX5-derived ROS oxidizes and activates local Src to promote malignancy of tumor cells. Signal Transduct Target Ther 5:139. https://doi.org/10.1038/s41392-020-0193-z
Chen J, Wang Y, Zhang W, Zhao D, Zhang L, Zhang J, Fan J, Zhan Q (2021) NOX5 mediates the crosstalk between tumor cells and cancer‐associated fibroblasts via regulating cytokine network. Clin Transl Med 11.https://doi.org/10.1002/ctm2.472
Chen Q, Wang Q, Zhu J, Xiao Q, Zhang L (2018) Reactive oxygen species: key regulators in vascular health and diseases. Br J Pharmacol 175:1279–1292. https://doi.org/10.1111/bph.13828
Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD (2001) Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269:131–140. https://doi.org/10.1016/s0378-1119(01)00449-8
Cortes A, Solas M, Pejenaute A, Abellanas MA, Garcia-Lacarte M, Aymerich MS, Marques J, Ramirez MJ, Zalba G (2021) Expression of endothelial NOX5 alters the integrity of the blood-brain barrier and causes loss of memory in aging mice. Antioxidants (Basel) 10. https://doi.org/10.3390/antiox10081311.
Cross AR, Segal AW (2004) The NADPH oxidase of professional phagocytes prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657:1–22. https://doi.org/10.1016/j.bbabio.2004.03.008
de Deken X, Corvilain B, Dumont JE, Miot F (2014) Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid Redox Signal 20:2776–2793. https://doi.org/10.1089/ars.2013.5602
de Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F (2000) Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275:23227–23233. https://doi.org/10.1074/jbc.M000916200
Deliyanti D, Alrashdi SF, Touyz RM, Kennedy CR, Jha JC, Cooper ME, Jandeleit-Dahm KA, Wilkinson-Berka JL (2020) Nox (NADPH Oxidase) 1, Nox4, and Nox5 promote vascular permeability and neovascularization in retinopathy. Hypertension 75:1091–1101. https://doi.org/10.1161/HYPERTENSIONAHA.119.14100
Dho SH, Kim JY, Kwon E-S, Lim JC, Park SS, Kwon KS (2015) NOX5-L can stimulate proliferation and apoptosis depending on its levels and cellular context determining cancer cell susceptibility to cisplatin. Oncotarget 6:39235–39246. https://doi.org/10.18632/oncotarget.5743
Dho SH, Kim JY, Lee KP, Kwon ES, Lim JC, Kim CJ, Jeong D, Kwon KS (2017) STAT5A-mediated NOX5-L expression promotes the proliferation and metastasis of breast cancer cells. Exp Cell Res 351:51–58. https://doi.org/10.1016/j.yexcr.2016.12.020
Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W (2006) cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in barrett esophageal adenocarcinoma cells. J Biol Chem 281:20368–20382. https://doi.org/10.1074/jbc.M603353200
Fulton DJ (2009) Nox5 and the regulation of cellular function. Antioxid Redox Signal 11:2443–2452. https://doi.org/10.1089/ARS.2009.2587
Furmanik M, Chatrou M, van Gorp R, Akbulut A, Willems B, Schmidt H, van Eys G, Bochaton-Piallat M-L, Proudfoot D, Biessen E, Hedin U, Perisic L, Mees B, Shanahan C, Reutelingsperger C, Schurgers L (2020) Reactive oxygen-forming Nox5 links vascular smooth muscle cell phenotypic switching and extracellular vesicle-mediated vascular calcification. Circ Res 127:911–927. https://doi.org/10.1161/CIRCRESAHA.119.316159
Gao X, Schottker B (2017) Reduction-oxidation pathways involved in cancer development: a systematic review of literature reviews. Oncotarget 8:51888–51906. https://doi.org/10.18632/oncotarget.17128
Garcia JG, Ansorena E, Milagro FI, Zalba G, de Miguel C (2021a) Endothelial Nox5 expression modulates glucose uptake and lipid accumulation in mice fed a high-fat diet and 3T3-L1 adipocytes treated with glucose and palmitic acid. Int J Mol Sci 22. https://doi.org/10.3390/ijms22052729.
Garcia JG, de Miguel C, Milagro FI, Zalba G, Ansorena E (2021b) Endothelial NOX5 expression modulates thermogenesis and lipolysis in mice fed with a high-fat diet and 3T3-L1 adipocytes through an Interleukin-6 dependent mechanism. Antioxidants (Basel) 11. https://doi.org/10.3390/antiox11010030.
Ge QM, Huang CM, Zhu XY, Bian F, Pan SM (2017) Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS One 12:e0173292. https://doi.org/10.1371/journal.pone.0173292
Geiszt M, Kopp JB, Varnai P, Leto TL (2000) Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 97:8010–8014. https://doi.org/10.1073/pnas.130135897
Ghanbari H, Keshtgar S, Kazeroni M (2018) Inhibition of the CatSper channel and NOX5 enzyme activity affects the functions of the progesterone-stimulated human sperm. Iran J Med Sci 43:18–25
Goncalves JDS, Carvalho FL, Coutinho I, Morais JCO, Fortunato RS (1992) Milito CB (2020) NADPH Oxidase 5 upregulation is associated with lymphoma aggressiveness. Rev Assoc Med Bras 66:210–215. https://doi.org/10.1590/1806-9282.66.2.210
Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R (2000) A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87:26–32. https://doi.org/10.1161/01.res.87.1.26
Grasberger H, El-Zaatari M, Dang DT, Merchant JL (2013) Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology 145:1045–1054. https://doi.org/10.1053/j.gastro.2013.07.011
Groemping Y, Rittinger K (2005) Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J 386:401–416. https://doi.org/10.1042/BJ20041835
Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG (2008) Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52:1803–1809. https://doi.org/10.1016/j.jacc.2008.07.063
Hahn NE, Meischl C, Kawahara T, Musters RJ, Verhoef VM, van der Velden J, Vonk AB, Paulus WJ, van Rossum AC, Niessen HW, Krijnen PA (2012) NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am J Pathol 180:2222–2229. https://doi.org/10.1016/j.ajpath.2012.02.018
Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM (2003) Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41:2164–2171. https://doi.org/10.1016/s0735-1097(03)00471-6
Ho F, Watson AMD, Elbatreek MH, Kleikers PWM, Khan W, Sourris KC, Dai A, Jha J, Schmidt HHHW, Jandeleit-Dahm KAM (2022) Endothelial reactive oxygen-forming NADPH oxidase 5 is a possible player in diabetic aortic aneurysm but not atherosclerosis. Sci Rep 12:11570. https://doi.org/10.1038/s41598-022-15706-5
Höll M, Koziel R, Schäfer G, Pircher H, Pauck A, Hermann M, Klocker H, Jansen-Dürr P, Sampson N (2016) ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol Carcinog 55:27–39. https://doi.org/10.1002/mc.22255
Holterman CE, Thibodeau JF, Towaij C, Gutsol A, Montezano AC, Parks RJ, Cooper ME, Touyz RM, Kennedy CR (2014) Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol 25:784–797. https://doi.org/10.1681/ASN.2013040371
Hu T, Ramachandrarao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K (2005) Reactive oxygen species production via NADPH oxidase mediates TGF-beta-induced cytoskeletal alterations in endothelial cells. Am J Physiol Renal Physiol 289:F816–F825. https://doi.org/10.1152/ajprenal.00024.2005
Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ (2007) Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem 282:6494–6507. https://doi.org/10.1074/jbc.M608966200
Javeshghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN (2002) Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 165:412–418. https://doi.org/10.1164/ajrccm.165.3.2103028
Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassegue B, Griendling KK (2008) Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45:329–335. https://doi.org/10.1016/j.freeradbiomed.2008.04.024
Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, Thallas-Bonke V, de Vos L, Holterman CE, Coughlan MT, Power DA, Skene A, Ekinci EI, Cooper ME, Touyz RM, Kennedy CR, Jandeleit-Dahm K (2017) NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes 66:2691–2703. https://doi.org/10.2337/db16-1585
Jha JC, Dai A, Garzarella J, Charlton A, Urner S, Ostergaard JA, Okabe J, Holterman CE, Skene A, Power DA, Ekinci EI, Coughlan MT, Schmidt H, Cooper ME, Touyz RM, Kennedy CR, Jandeleit-Dahm K (2022) Independent of Renox, NOX5 promotes renal inflammation and fibrosis in diabetes by activating ROS-sensitive pathways. Diabetes 71:1282–1298. https://doi.org/10.2337/db21-1079
Jha JC, Dai A, Garzarella J, Charlton A, Urner S, Østergaard JA, Okabe J, Holterman CE, Skene A, Power DA, Ekinci EI, Coughlan MT, Schmidt HHHW, Cooper ME, Touyz RM, Kennedy CR, Jandeleit-Dahm K (2022) Independent of Renox, NOX5 promotes renal inflammation and fibrosis in diabetes by activating ROS-sensitive pathways. Diabetes 71:1282–1298. https://doi.org/10.2337/db21-1079
Jha JC, Watson AMD, Mathew G, de Vos LC, Jandeleit-Dahm K (2017) The emerging role of NADPH oxidase NOX5 in vascular disease. Clin Sci (Lond) 131:981–990. https://doi.org/10.1042/CS20160846
Kalinina EV, Andreev YA, Petrova AS, Lubova KI, Shtil’ AA, Chernov NN, Novichkova MD, Nurmuradov NK (2018) Redox-dependent expression of genes encoding NADPH oxidase 5 and the key antioxidant enzymes during formation of drug resistance of tumor cells to cisplatin. Bull Exp Biol Med 165:678–681. https://doi.org/10.1007/s10517-018-4240-5
Kawahara T, Lambeth JD (2008) Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell 19:4020–4031. https://doi.org/10.1091/mbc.E07-12-1223
Kawahara T, Quinn MT, Lambeth JD (2007) Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol 7:109. https://doi.org/10.1186/1471-2148-7-109
Kleikers PW, Dao VT, Gob E, Hooijmans C, Debets J, van Essen H, Kleinschnitz C, Schmidt HH (2014) SFRR-E Young Investigator AwardeeNOXing out stroke: identification of NOX4 and 5 as targets in blood-brain-barrier stabilisation and neuroprotection. Free Radic Biol Med 75(Suppl 1):S16. https://doi.org/10.1016/j.freeradbiomed.2014.10.593
Lamirande E, Gagnon C (1995) Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod 10(Suppl 1):15–21. https://doi.org/10.1093/humrep/10.suppl_1.15
Laurent V, Toulet A, Attané C, Milhas D, Dauvillier S, Zaidi F, Clement E, Cinato M, le Gonidec S, Guérard A, Lehuédé C, Garandeau D, Nieto L, Renaud-Gabardos E, Prats A-C, Valet P, Malavaud B, Muller C (2019) Periprostatic adipose tissue favors prostate cancer cell invasion in an obesity-dependent manner: role of oxidative stress. Mol Cancer Res 17:821–835. https://doi.org/10.1158/1541-7786.MCR-18-0748
Lee ES, Kim HM, Lee SH, Ha KB, Bae YS, Lee SJ, Moon SH, Lee EY, Lee J-H, Chung CH (2020) APX-115, a pan-NADPH oxidase inhibitor, protects development of diabetic nephropathy in podocyte specific NOX5 transgenic mice. Free Radic Biol Med 161:92–101. https://doi.org/10.1016/j.freeradbiomed.2020.09.024
Leseney AM, Deme D, Legue O, Ohayon R, Chanson P, Sales JP, Carvalho DP, Dupuy C, Virion A (1999) Biochemical characterization of a Ca2+/NAD(P)H-dependent H2O2 generator in human thyroid tissue. Biochimie 81:373–380. https://doi.org/10.1016/s0300-9084(99)80084-4
Li D, Cao W (2016) Bile acid receptor TGR5, NADPH Oxidase NOX5-S and CREB mediate bile acid-induced DNA damage in Barrett’s esophageal adenocarcinoma cells. Sci Rep 6:31538. https://doi.org/10.1038/srep31538
Liang S, Kisseleva T, Brenner DA (2016) The Role of NADPH Oxidases (NOXs) in liver fibrosis and the activation of myofibroblasts. Front Physiol 7:17. https://doi.org/10.3389/fphys.2016.00017
Lobysheva I, Rath G, Sekkali B, Bouzin C, Feron O, Gallez B, Dessy C, Balligand JL (2011) Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol 31:2098–2105. https://doi.org/10.1161/ATVBAHA.111.230623
Madrigal-Matute J, Fernandez-Garcia CE, Gomez-Guerrero C, Lopez-Franco O, Munoz-Garcia B, Egido J, Blanco-Colio LM, Martin-Ventura JL (2012) HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc Res 95:116–123. https://doi.org/10.1093/cvr/cvs158
Magnani F, Nenci S, Millana Fananas E, Ceccon M, Romero E, Fraaije MW, Mattevi A (2017) Crystal structures and atomic model of NADPH oxidase. Proc Natl Acad Sci U S A 114:6764–6769. https://doi.org/10.1073/pnas.1702293114
Mahbouli S, der Vartanian A, Ortega S, Rouge S, Vasson MP, Rossary A (2017) Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol Rep 38:3254–3264. https://doi.org/10.3892/or.2017.6009
Manea SA, Todirita A, Raicu M, Manea A (2014) C/EBP transcription factors regulate NADPH oxidase in human aortic smooth muscle cells. J Cell Mol Med 18:1467–1477. https://doi.org/10.1111/jcmm.12289
Marques J, Cortes A, Pejenaute A, Ansorena E, Abizanda G, Prosper F, Martinez-Irujo JJ, Miguel C, Zalba G (2020a) Induction of Cyclooxygenase-2 by overexpression of the human NADPH oxidase 5 (NOX5) gene in aortic endothelial cells. Cells 9. https://doi.org/10.3390/cells9030637.
Marques J, Cortes A, Pejenaute A, Zalba G (2020) Implications of NADPH oxidase 5 in vascular diseases. Int J Biochem Cell Biol 128:105851. https://doi.org/10.1016/j.biocel.2020.105851
Marzaioli V, Hurtado-Nedelec M, Pintard C, Tlili A, Marie JC, Monteiro RC, Gougerot-Pocidalo MA, Dang PM, El-Benna J (2017) NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood 130:1734–1745. https://doi.org/10.1182/blood-2016-10-746347
Miguel-Jimenez S, Pina-Beltran B, Gimeno-Martos S, Carvajal-Serna M, Casao A, Perez-Pe R (2021) NADPH oxidase 5 and melatonin: involvement in ram sperm capacitation. Front Cell Dev Biol 9:655794. https://doi.org/10.3389/fcell.2021.655794
Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM (2010) Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106:1363–1373. https://doi.org/10.1161/CIRCRESAHA.109.216036
Montezano AC, de Lucca Camargo L, Persson P, Rios FJ, Harvey AP, Anagnostopoulou A, Palacios R, Gandara ACP, Alves-Lopes R, Neves KB, Dulak-Lis M, Holterman CE, de Oliveira PL, Graham D, Kennedy C, Touyz RM (2018) NADPH oxidase 5 is a pro-contractile Nox isoform and a point of cross-talk for calcium and redox signaling-implications in vascular function. J Am Heart Assoc 7. https://doi.org/10.1161/JAHA.118.009388.
Musset B, Clark RA, DeCoursey TE, Petheo GL, Geiszt M, Chen Y, Cornell JE, Eddy CA, Brzyski RG, el Jamali A (2012) NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem 287:9376–9388. https://doi.org/10.1074/jbc.M111.314955
Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD (2014) Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53:5111–5120. https://doi.org/10.1021/bi500331y
Pandey D, Fulton DJ (2011) Molecular regulation of NADPH oxidase 5 via the MAPK pathway. Am J Physiol Heart Circ Physiol 300:H1336–H1344. https://doi.org/10.1152/ajpheart.01163.2010
Pandey D, Gratton JP, Rafikov R, Black SM, Fulton DJ (2011) Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol Pharmacol 80:407–415. https://doi.org/10.1124/mol.110.070193
Pandey D, Patel A, Patel V, Chen F, Qian J, Wang Y, Barman SA, Venema RC, Stepp DW, Rudic RD, Fulton DJ (2012) Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. Am J Physiol Heart Circ Physiol 302:H1919–H1928. https://doi.org/10.1152/ajpheart.00910.2011
Peng JJ, Liu B, Xu JY, Peng J, Luo XJ (2017) NADPH oxidase: its potential role in promotion of pulmonary arterial hypertension. Naunyn Schmiedebergs Arch Pharmacol 390:331–338. https://doi.org/10.1007/s00210-017-1359-2
Petheo GL, Kerekes A, Mihalffy M, Donko A, Bodrogi L, Skoda G, Barath M, Hoffmann OI, Szeles Z, Balazs B, Sirokmany G, Fabian JR, Toth ZE, Baksa I, Kacskovics I, Hunyady L, Hiripi L, Bosze Z, Geiszt M (2021) Disruption of the NOX5 gene aggravates atherosclerosis in rabbits. Circ Res 128:1320–1322. https://doi.org/10.1161/CIRCRESAHA.120.318611
Pi X, Xie L, Portbury AL, Kumar S, Lockyer P, Li X, Patterson C (2014) NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1alpha-stimulated angiogenesis. Arterioscler Thromb Vasc Biol 34:2023–2032. https://doi.org/10.1161/ATVBAHA.114.303733
Poznyak A v, Grechko A v, Orekhova VA, Khotina V, Ivanova EA, Orekhov AN (2020) NADPH oxidases and their role in atherosclerosis. Biomedicines 8. https://doi.org/10.3390/biomedicines8070206.
Prior KK, Leisegang MS, Josipovic I, Lowe O, Shah AM, Weissmann N, Schroder K, Brandes RP (2016) CRISPR/Cas9-mediated knockout of p22phox leads to loss of Nox1 and Nox4, but not Nox5 activity. Redox Biol 9:287–295. https://doi.org/10.1016/j.redox.2016.08.013
Reinehr R, Becker S, Eberle A, Grether-Beck S, Haussinger D (2005) Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J Biol Chem 280:27179–27194. https://doi.org/10.1074/jbc.M414361200
Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G, Lu J, Antony S, Doroshow JH (2015) NADPH oxidases and cancer. Clin Sci (Lond) 128:863–875. https://doi.org/10.1042/CS20140542
Royer-Pokora B, Kunkel LM, Monaco AP, Goff SC, Newburger PE, Baehner RL, Cole FS, Curnutte JT, Orkin SH (1986) Cloning the gene for an inherited human disorder -chronic granulomatous disease- on the basis of its chromosomal location. Nature 322:32–38. https://doi.org/10.1038/322032a0
Sabeur K, Ball BA (2007) Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction 134:263–270. https://doi.org/10.1530/REP-06-0120
Setyawan EMN, Kim MJ, Oh HJ, Kim GA, Jo YK, Lee SH, Choi YB, Lee BC (2016) Spermine reduces reactive oxygen species levels and decreases cryocapacitation in canine sperm cryopreservation. Biochem Biophys Res Commun 479:927–932. https://doi.org/10.1016/j.bbrc.2016.08.091
Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H (2001) A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276:1417–1423. https://doi.org/10.1074/jbc.M007597200
Si J, Behar J, Wands J, Beer DG, Lambeth D, Chin YE, Cao W (2008) STAT5 mediates PAF-induced NADPH oxidase NOX5-S expression in Barrett’s esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol 294:G174–G183. https://doi.org/10.1152/ajpgi.00291.2007
Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, Krause KH (2005) Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol 207:164–176. https://doi.org/10.1002/path.1824
Tarafdar A, Pula G (2018) The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci 19. https://doi.org/10.3390/ijms19123824.
Tirone F, Radu L, Craescu CT, Cox JA (2010) Identification of the binding site for the regulatory calcium-binding domain in the catalytic domain of NOX5. Biochemistry 49:761–771. https://doi.org/10.1021/bi901846y
Touyz RM, Anagnostopoulou A, Camargo LL, Rios FJ, Montezano AC (2019) Vascular biology of superoxide-generating NADPH oxidase 5-implications in hypertension and cardiovascular disease. Antioxid Redox Signal 30:1027–1040. https://doi.org/10.1089/ars.2018.7583
Touyz RM, Anagnostopoulou A, Rios F, Montezano AC, Camargo LL (2019) NOX5: molecular biology and pathophysiology. Exp Physiol 104:605–616. https://doi.org/10.1113/EP086204
Ueno N, Takeya R, Miyano K, Kikuchi H, Sumimoto H (2005) The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem 280:23328–23339. https://doi.org/10.1074/jbc.M414548200
Wang D, de Deken X, Milenkovic M, Song Y, Pirson I, Dumont JE, Miot F (2005) Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J Biol Chem 280:3096–3103. https://doi.org/10.1074/jbc.M407709200
Wu J, Lu K, Zhu M, Xie X, Ding Y, Shao X, Chen Y, Liu J, Xu M, Xu Y, Zhou J, Shen X, Zhu C (2020) miR-485 suppresses inflammation and proliferation of mesangial cells in an in vitro model of diabetic nephropathy by targeting NOX5. Biochem Biophys Res Commun 521:984–990. https://doi.org/10.1016/j.bbrc.2019.11.020
Xiong Y, Chen L, Yu T, Yan C, Zhou W, Cao F, You X, Zhang Y, Sun Y, Liu J, Xue H, Hu Y, Chen D, Mi B, Liu G (2020) Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany NY) 12:8968–8986. https://doi.org/10.18632/aging.103143
Yeung KR, Chiu CL, Pidsley R, Makris A, Hennessy A, Lind JM (2016) DNA methylation profiles in preeclampsia and healthy control placentas. Am J Physiol Heart Circ Physiol 310:H1295–H1303. https://doi.org/10.1152/ajpheart.00958.2015
Zhao G-J, Zhao C-L, Ouyang S, Deng K-Q, Zhu L, Montezano AC, Zhang C, Hu F, Zhu X-Y, Tian S, Liu X, Ji Y-X, Zhang P, Zhang X-J, She Z-G, Touyz RM, Li H (2020) Ca 2+ -dependent NOX5 (NADPH oxidase 5) exaggerates cardiac hypertrophy through reactive oxygen species production. Hypertension 76:827–838. https://doi.org/10.1161/HYPERTENSIONAHA.120.15558
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Spanish Ministry of Economy, Industry and Competitiveness (SAF2013-49088-R, SAF2016-79151-R); the Spanish Ministry of Science and Innovation (FIS PI22/01450) the Department of Health of the Government of Navarra (2021/40); and the Centro de Investigación Biomédica en Red de la Fisiopatología de la Obesidad y Nutrición (CIBERobn). J.G has received predoctoral grants from Asociación de Amigos of the University of Navarra and “La Caixa” Foundation.
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used. Writing—original draft preparation: J.G, E.A. and F.M.; writing—review and editing: J.G, E.A, CdM, G.Z, and F.M; figure preparation: I.I; funding acquisition: G.Z and F.M. “All authors have read and agreed to the published the version of the manuscript.”
Corresponding author
Ethics declarations
Research involving human participants and/or animals
NA
Informed consent
NA
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keypoints
- NOX5 levels increase in the body as a response to stress.
- NOX5 has been associated with the development of pathologies and metabolic complications.
- NOX5 might prepare the body for stressful situations.
- Endothelial NOX5 activity promotes thermogenesis and lipolysis in the adipose tissue of knock-in transgenic mice by activating IL-6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
García, J.G., Ansorena, E., Izal, I. et al. Structure, regulation, and physiological functions of NADPH oxidase 5 (NOX5). J Physiol Biochem 79, 383–395 (2023). https://doi.org/10.1007/s13105-023-00955-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00955-3