Abstract
Background
Pancreatic ductal adenocarcinoma (PDAC) is one of the most fatal malignant tumors of the digestive system. Many patients are diagnosed at an advanced stage and lose eligibility for surgery. Moreover, there are few effective methods for treating pancreatic ductal cell carcinoma. Increasing attention has been given to microRNAs (miRNAs) and their regulatory roles in tumor progression. In this study, we investigated the effects of exosomes extracted from human umbilical cord mesenchymal stem cells (HUCMSCs) carrying hsa-miRNA-128-3p on pancreatic cancer cells.
Methods
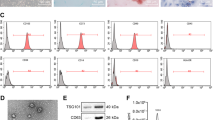
Based on existing experimental and database information, we selected Galectin-3, which is associated with pancreatic cancer, and the corresponding upstream hsa-miRNA-128-3p. We extracted HUCMSCs from a fresh umbilical cord, hsa-miRNA-128-3p was transfected into HUCMSCs, and exosomes containing hsa-miRNA-128-3p were extracted and collected. The effect of exosomes rich in hsa-miRNA-128-3p on pancreatic cancer cells was analyzed.
Results
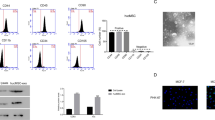
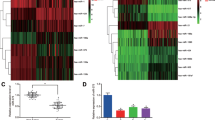
The expression of Galectin-3 in normal pancreatic duct epithelial cells was significantly lower than that in PDAC cell lines. We successfully extracted HUCMSCs from the umbilical cord and transfected hsa-miRNA-128-3p into HUCMSCs. Then we demonstrated that HUCMSC-derived exosomes with hsa-miRNA-128-3p could suppress the proliferation, invasion, and migration of PANC-1 cells in vitro by targeting Galectin-3.
Conclusion
Hsa-miRNA-128-3p could be considered as a potential therapy for pancreatic cancer. We provided a new idea for targeted therapy of PDAC.












Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. https://doi.org/10.1016/S0140-6736(20)30974-0.
Zhou B, Xu JW, Cheng YG, et al. Early detection of pancreatic cancer: Where are we now and where are we going? Int J Cancer. 2017;141(2):231–41. https://doi.org/10.1002/ijc.30670.
Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–94. https://doi.org/10.1007/s10456-014-9420-y.
Desai PB. Understanding the biology of cancer—has this any impact on treatment. J Cancer Res Clin Oncol. 1994;120(4):193–9. https://doi.org/10.1007/bf01372555.
Cetin I, Topcul M. Cancer stem cells in oncology. Eur Surg Res. 2012;17(4):644–8. https://doi.org/10.1159/000339610.
Xie X, Wu H, Li M, et al. Progress in the application of exosomes as therapeutic vectors in tumor-targeted therapy. Cytotherapy. 2019;21(5):509–24. https://doi.org/10.1016/j.jcyt.2019.01.001.
Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev Rep. 2011;7(1):195–207. https://doi.org/10.1007/s12015-010-9168-8.
Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–7. https://doi.org/10.1634/stemcells.2004-0013.
Li DR, Cai JH. Methods of isolation, expansion, differentiating induction and preservation of human umbilical cord mesenchymal stem cells. Chin Med J. 2012;125(24):4504–10. https://doi.org/10.3760/cma.j.issn.0366-6999.2012.24.032.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–12. https://doi.org/10.1007/s10571-016-0366-z.
Khan S, Brougham CL, Ryan J, et al. miR-379 regulates cyclin b1 expression and is decreased in breast cancer. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0068753.
Chen JS, Li HS, Huang JQ, et al. MicroRNA-379-5p inhibits tumor invasion and metastasis by targeting FAK/AKT signaling in hepatocellular carcinoma. Cancer Lett. 2016;375(1):73–83. https://doi.org/10.1016/j.canlet.2016.02.043.
Li Z, Shen JX, Chan MTV, et al. MicroRNA-379 suppresses osteosarcoma progression by targeting PDK1. J Cell Mol Med. 2017;21(2):315–23. https://doi.org/10.1111/jcmm.12966.
O’Brien KP, Khan S, Gilligan KE, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018. https://doi.org/10.1038/s41388-017-0116-9.
Li Z, Ni J, Song D, et al. Regulatory mechanism of microRNA-128 in osteosarcoma tumorigenesis and evolution through targeting SASH1. Oncol Lett. 2018;15(6):8687–94. https://doi.org/10.3892/ol.2018.8397.
Hoyer KK, Pang M, Gui D, et al. An anti-apoptotic role for galectin-3 in diffuse large B-cell lymphomas. Am J Pathol. 2004;164(3):893–902. https://doi.org/10.1016/s0002-9440(10)63177-x.
Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156(3):899–909. https://doi.org/10.1016/s0002-9440(10)64959-0.
Song SM, Ji BA, Ramachandran V, et al. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0042699.
Merlin J, Stechly L, de Beauce S, et al. Galectin-3 regulates MUC1 and EGFR cellular distribution and EGFR downstream pathways in pancreatic cancer cells. Oncogene. 2011;30(22):2514–25. https://doi.org/10.1038/onc.2010.631.
Yao YL, Zhou LS, Liao WF, et al. HH1–1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr Polym. 2019;204:111–23. https://doi.org/10.1016/j.carbpol.2018.10.008.
Xie L, Ni WK, Chen XD, et al. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol. 2012;138(6):1035–43. https://doi.org/10.1007/s00432-012-1178-2.
Luo G, Jin K, Deng S, et al. Roles of CA19–9 in pancreatic cancer: biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188409. https://doi.org/10.1016/j.bbcan.2020.188409.
Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet (London, England). 2016;388(10039):73–85. https://doi.org/10.1016/S0140-6736(16)00141-0.
Gaida MM, Bach ST, Guenther F, et al. Expression of galectin-3 in pancreatic ductal adenocarcinoma. Pathol Oncol Res. 2012;18(2):299–307. https://doi.org/10.1007/s12253-011-9444-1.
Yi N, Zhao X, Ji J, et al. Serum galectin-3 as a biomarker for screening, early diagnosis, prognosis and therapeutic effect evaluation of pancreatic cancer. J Cell Mol Med. 2020. https://doi.org/10.1111/jcmm.15775.
Bieback K, Netsch P. Isolation, culture, and characterization of human umbilical cord blood-derived mesenchymal stromal cells. Methods Mol Biol (Clifton, NJ). 2016;1416:245–58. https://doi.org/10.1007/978-1-4939-3584-0_14.
Kogure T, Lin WL, Yan IK, et al. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–48. https://doi.org/10.1002/hep.24504.
Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release Off J Control Release Soc. 2013;172(3):962–74. https://doi.org/10.1016/j.jconrel.2013.09.015.
Saijo N. Progress in cancer chemotherapy with special stress on molecular-targeted therapy. Jap J Clin Oncol. 2010;40(9):855–62. https://doi.org/10.1093/jjco/hyq035.
Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol. 2008;26(11):1774–7. https://doi.org/10.1200/jco.2007.15.7438.
Reichert JM, Valge-Archer VE. Outlook—development trends for monoclonal antibody cancer therapeutics. Nat Rev Drug Discov. 2007;6(5):349–56. https://doi.org/10.1038/nrd2241.
Berger M, Shankar V, Vafai A. Therapeutic applications of monoclonal antibodies. Am J Med Sci. 2002;324(1):14–30. https://doi.org/10.1097/00000441-200207000-00004.
Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–87. https://doi.org/10.1038/nrc3236.
Firer MA, Gellerman G. Targeted drug delivery for cancer therapy: the other side of antibodies. J Hematol Oncol. 2012. https://doi.org/10.1186/1756-8722-5-70.
Lv H, Zhang S, Wang B, et al. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–9. https://doi.org/10.1016/j.jconrel.2006.04.014.
Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–58. https://doi.org/10.1038/nrg1066.
Harris JM, Martin NE, Modi M. Pegylation—a novel process for modifying pharmacokinetics. Clin Pharmacokinet. 2001;40(7):539–51. https://doi.org/10.2165/00003088-200140070-00005.
Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44(11):2060–4. https://doi.org/10.1016/j.biocel.2012.08.007.
van den Boorn JG, Schlee M, Coch C, et al. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29(4):325–6. https://doi.org/10.1038/nbt.1830.
Alvarez-Erviti L, Seow YQ, Yin HF, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341-U179. https://doi.org/10.1038/nbt.1807.
Holder B, Jones T, Shimizu VS, et al. Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic. 2016;17(2):168–78. https://doi.org/10.1111/tra.12352.
Shi RJ, Zhao LB, Cai WB, et al. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem Biophys Res Commun. 2017;483(1):602–8. https://doi.org/10.1016/j.bbrc.2016.12.097.
Wang M, Zhao C, Shi H, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110(5):1199–210. https://doi.org/10.1038/bjc.2014.14.
Matsuzaki J, Suzuki H. Role of microRNAs-221/222 in digestive systems. J Clin Med. 2015;4(8):1566–77. https://doi.org/10.3390/jcm4081566.
Ma M, Chen SL, Liu Z, et al. miRNA-221 of exosomes originating from bone marrow mesenchymal stem cells promotes oncogenic activity in gastric cancer. Onco Targets Ther. 2017;10:4161–71. https://doi.org/10.2147/ott.S143315.
Acknowledgements
This study was supported by grants from Natural Science Foundation of Jiangsu Province (no.BK20211105), the Key Research and Development Plan of Jiangsu Province (no.BE2019692), Postdoctoral Science Foundation of China (Grant No. 2019M661909), The Health Project of Jiangsu Province (Grant No. H2019072), the Social Development Foundation of Nantong City (Grant Nos. MS12020018, MSZ20076, JCZ20065 and MSZ19177).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, X., Ji, J., Chen, X. et al. Human umbilical cord mesenchymal stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin Transl Oncol 24, 517–531 (2022). https://doi.org/10.1007/s12094-021-02705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02705-7