Abstract
Purpose
Glutamine plays an important role in tumor metabolism and progression. This research aimed to find out how Gln exert their effects on laryngeal squamous cell carcinoma (LSCC).
Methods
Cell proliferation was measured by CCK8 and EdU assay, mitochondrial bioenergetic activity was measured by mitochondrial stress tests. Gene expression profiling was revealed by RNA sequencing and validated by RT-qPCR. In LSCC patients, protein expression in tumor and adjacent tissues was examined and scored by IHC staining. RNAi was performed by stably expressed shRNA in TU177 cells. In vivo tumor growth analysis was performed using a nude mouse tumorigenicity model.
Results
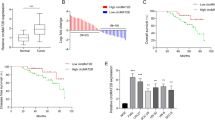
Gln deprivation suppressed TU177 cell proliferation, which was restored by αKG supplementation. By transcriptomic analysis, we identified CECR2, which encodes a histone acetyl-lysine reader, as the downstream target gene for Gln and αKG. In LSCC patients, the expression of CECR2 in tumors was lower than adjacent tissues. Furthermore, deficiency of CECR2 promoted tumor cell growth both in vitro and in vivo, suggesting it has tumor suppressor effects. Besides, cell proliferation inhibited by Gln withdrawal could be restored by CECR2 depletion, and the proliferation boosted by αKG supplementation could be magnified either, suggested that CECR2 feedback suppressed Gln and αKG’s effect on tumor growth. Transcriptomic profiling revealed CECR2 regulated the expression of a series of genes involved in tumor progression.
Conclusion
We confirmed the Gln-αKG-CECR2 axis contributes to tumor growth in LSCC. This finding provided a potential therapeutic opportunity for the use of associated metabolites as a potential treatment for LSCC.







Similar content being viewed by others
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
References
Lionello M, Staffieri A, Marioni G. Potential prognostic and therapeutic role for angiogenesis markers in laryngeal carcinoma. Acta Otolaryngol. 2012;132(6):574–82. https://doi.org/10.3109/00016489.2011.652308.
Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269–82. https://doi.org/10.1038/nrc.2018.11.
Yokota T, Homma A, Kiyota N, Tahara M, Hanai N, Asakage T, et al. Immunotherapy for squamous cell carcinoma of the head and neck. Jpn J Clin Oncol. 2020;50(10):1089–96. https://doi.org/10.1093/jjco/hyaa139.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. https://doi.org/10.3322/caac.21660.
Qureshi MM, Oladeru OT, Lam CM, Dyer MA, Mak KS, Hirsch AE, et al. Disparities in laryngeal cancer treatment and outcomes: an analysis by hospital safety-net burden. Laryngoscope. 2021. https://doi.org/10.1002/lary.29416.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–34. https://doi.org/10.1038/nrc.2016.71.
Smith B, Schafer XL, Ambeskovic A, Spencer CM, Land H, Munger J. Addiction to coupling of the Warburg effect with glutamine catabolism in cancer cells. Cell Rep. 2016;17(3):821–36. https://doi.org/10.1016/j.celrep.2016.09.045.
Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. https://doi.org/10.1016/j.cmet.2015.12.006.
Bott AJ, Shen J, Tonelli C, Zhan L, Sivaram N, Jiang YP, et al. Glutamine anabolism plays a critical role in pancreatic cancer by coupling carbon and nitrogen metabolism. Cell Rep. 2019;29(5):1287-98.e6. https://doi.org/10.1016/j.celrep.2019.09.056.
Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123(9):3678–84. https://doi.org/10.1172/jci69600.
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69(20):7986–93. https://doi.org/10.1158/0008-5472.Can-09-2266.
Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26(9):877–90. https://doi.org/10.1101/gad.189365.112.
Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254(8):2669–76.
Cacace A, Sboarina M, Vazeille T, Sonveaux P. Glutamine activates STAT3 to control cancer cell proliferation independently of glutamine metabolism. Oncogene. 2017;36(15):2074–84. https://doi.org/10.1038/onc.2016.364.
TeSlaa T, Chaikovsky AC, Lipchina I, Escobar SL, Hochedlinger K, Huang J, et al. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24(3):485–93. https://doi.org/10.1016/j.cmet.2016.07.002.
Liu PS, Wang H, Li X, Chao T, Teav T, Christen S, et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat Immunol. 2017;18(9):985–94. https://doi.org/10.1038/ni.3796.
Tran TQ, Hanse EA, Habowski AN, Li H, Gabra MBI, Yang Y, et al. α-Ketoglutarate attenuates Wnt signaling and drives differentiation in colorectal cancer. Nat Cancer. 2020;1(3):345–58. https://doi.org/10.1038/s43018-020-0035-5.
Morris JPt, Yashinskie JJ, Koche R, Chandwani R, Tian S, Chen CC, , et al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature. 2019;573(7775):595–9. https://doi.org/10.1038/s41586-019-1577-5.
Wang X, Liu R, Qu X, Yu H, Chu H, Zhang Y, et al. α-Ketoglutarate-activated NF-κB signaling promotes compensatory glucose uptake and brain tumor development. Mol Cell. 2019;76(1):148-62.e7. https://doi.org/10.1016/j.molcel.2019.07.007.
Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I, et al. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer. 2011;117(13):2926–38. https://doi.org/10.1002/cncr.25868.
Dawe CE, Kooistra MK, Fairbridge NA, Pisio AC, McDermid HE. Role of chromatin remodeling gene Cecr2 in neurulation and inner ear development. Dev Dyn. 2011;240(2):372–83. https://doi.org/10.1002/dvdy.22547.
Thompson PJ, Norton KA, Niri FH, Dawe CE, McDermid HE. CECR2 is involved in spermatogenesis and forms a complex with SNF2H in the testis. J Mol Biol. 2012;415(5):793–806. https://doi.org/10.1016/j.jmb.2011.11.041.
Banting GS, Barak O, Ames TM, Burnham AC, Kardel MD, Cooch NS, et al. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum Mol Genet. 2005;14(4):513–24. https://doi.org/10.1093/hmg/ddi048.
Park SG, Lee D, Seo HR, Lee SA, Kwon J. Cytotoxic activity of bromodomain inhibitor NVS-CECR2-1 on human cancer cells. Sci Rep. 2020;10(1):16330. https://doi.org/10.1038/s41598-020-73500-7.
Lee JH, Liu R, Li J, Zhang C, Wang Y, Cai Q, et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun. 2017;8(1):949. https://doi.org/10.1038/s41467-017-00906-9.
Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8(12):957–67. https://doi.org/10.1038/nrc2523.
Lee SK, Park EJ, Lee HS, Lee YS, Kwon J. Genome-wide screen of human bromodomain-containing proteins identifies Cecr2 as a novel DNA damage response protein. Mol Cells. 2012;34(1):85–91. https://doi.org/10.1007/s10059-012-0112-4.
McCrimmon A, Domondon M, Sultanova RF, Ilatovskaya DV, Stadler K. Comprehensive assessment of mitochondrial respiratory function in freshly isolated nephron segments. Am J Physiol Renal Physiol. 2020;318(5):F1237–45. https://doi.org/10.1152/ajprenal.00031.2020.
Beirowski B. Measuring bioenergetic signatures of peripheral nerve segments by extracellular flux analysis. Methods Mol Biol. 2020;2143:191–203. https://doi.org/10.1007/978-1-0716-0585-1_15.
Luz AL, Smith LL, Rooney JP, Meyer J. Seahorse Xfe 24 extracellular flux analyzer-based analysis of cellular respiration in Caenorhabditis elegans. Curr Protoc Toxicol. 2015;66:25.7.1-15. https://doi.org/10.1002/0471140856.tx2507s66.
Song L, Zhang S, Yu S, Ma F, Wang B, Zhang C, et al. Cellular heterogeneity landscape in laryngeal squamous cell carcinoma. Int J Cancer. 2020;147(10):2879–90. https://doi.org/10.1002/ijc.33192.
Pan M, Reid MA, Lowman XH, Kulkarni RP, Tran TQ, Liu X, et al. Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat Cell Biol. 2016;18(10):1090–101. https://doi.org/10.1038/ncb3410.
Sun N, Liang Y, Chen Y, Wang L, Li D, Liang Z, et al. Glutamine affects T24 bladder cancer cell proliferation by activating STAT3 through ROS and glutaminolysis. Int J Mol Med. 2019;44(6):2189–200. https://doi.org/10.3892/ijmm.2019.4385.
Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–5. https://doi.org/10.1038/nature12040.
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–6. https://doi.org/10.1038/nature13981.
Chen PH, Shen WL, Shih CM, Ho KH, Cheng CH, Lin CW, et al. The CHAC1-inhibited Notch3 pathway is involved in temozolomide-induced glioma cytotoxicity. Neuropharmacology. 2017;116:300–14. https://doi.org/10.1016/j.neuropharm.2016.12.011.
Joo NE, Ritchie K, Kamarajan P, Miao D, Kapila YL. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012;1(3):295–305. https://doi.org/10.1002/cam4.35.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Funding
This study was supported by National Natural Science Foundation of China (No. 81971240 to Liu F., No. 81770988 and 81970869 to Yi H.L., No. 81770987 to Guan J.); Shanghai Municipal Commission of Science and Technology (No.18DZ2260200). Jinan Science and Technology Development Program (No. 201907018 to Zhang N.) and Shandong Provincial Key Research and Development Program (No. 2017G006037 to Zhang N.)
Author information
Authors and Affiliations
Contributions
X.T.W., H.L.Y., J.G. and F.L. designed experiments. X.T.W., C.X. performed experiments and analyzed data. S.M.W., W.J.H. and Y.N.L. contributed to data collection, data interpretation. N.N.L., Z.F.G., F.W. and N.Z. contributed to protocol development. X.T.W., H.L.Y and F.L. prepared the figures and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The experimental protocol was established, according to the ethical guidelines of the Declaration of Helsinki (1964) and was approved by Chinese Clinical Trial Registry. The approval number is ChiCTR2000040554.
Consent to participate
Informed consents were obtained from all patients for the use of their samples.
Consent for publication
All authors have read and approved this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12094_2021_2603_MOESM1_ESM.png
Supplementary file1 Supplementary Fig. 1 Expression of relevant genes in single cell RNA(scRNA) sequencing in LSCC. a t‐SNE plot of the LSCC cell clusters in global view and the identification of cell‐class composition. b–f Expression of key enzymes, including IDH1 (b), IDH2 (c), GLS (d), PPAT (e) and GLS2 (f), required for Gln and αKG in LSCC. g–i Expression of candidate genes, CHAC1 (g), ULBP1 (h) and SLFN5 (i), in LSCC. (PNG 514 KB)
Rights and permissions
About this article
Cite this article
Wang, X., Xu, C., Wang, S. et al. A novel tumor suppressor CECR2 down regulation links glutamine metabolism contributes tumor growth in laryngeal squamous cell carcinoma. Clin Transl Oncol 23, 1942–1954 (2021). https://doi.org/10.1007/s12094-021-02603-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02603-y