Abstract
Purpose
This project aimed to survey the clinical characteristics and survivals of hyperprogressive disease (HPD) mediated by immune checkpoint inhibitors (ICIs) in an attempt to explore the potential predictors.
Methods
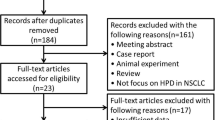
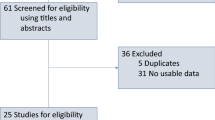
After searching PubMed, MEDLINE, Google Scholar and Cochrane Library databases, 12 studies incorporating 1766 individuals were enrolled. The data were analyzed with Review manager 5.3 software.
Results
The results revealed HPD correlated with previous metastatic sites > 2 (OR = 1.86, 95% CI 1.33–2.59, P = 0.0003), liver metastasis (OR = 3.35, 95% CI 2.09–5.35, P < 0.00001), Royal Marsden Hospital (RMH) score ≥ 2 (OR = 2.80, 95% CI 1.85–4.23, P < 0.00001), higher ECOG PS (OR = 1.60, 95% CI 1.13–2.27, P = 0.008) and LDH > upper limits of normal (ULN) (OR = 2.32, 95% CI 1.51–3.58, P = 0.0001). Instead, HPD was unrelated to gender, age, smoking status, PD-L1 expression, therapy, neutrophil-to-lymphocyte ratio, the histology, the status of EGFR, ALK and KRAS in non-small cell lung cancer and HER-2 expression in advanced gastric cancer. Moreover, HPD was evidently correlated with a shorter OS (HR = 2.92, 95% CI 1.79–4.76, P < 0.0001) and PFS (HR = 3.62, 95% CI 2.79–4.68, P < 0.00001). The same phenomena existed in stratified studies based on study regions and tumor types.
Conclusions
This study demonstrated that HPD was related to the number of prior metastatic sites > 2, liver metastasis, RMH score ≥ 2, higher ECOG PS score and LDH > ULN. Moreover, HPD was correlated with a poor OS and PFS in patients following ICI therapy.








Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Freddie B, Jacques F, Isabelle S, Siegel Rebecca L, Torre Lindsey A, Ahmedin J. Global Cancer Statistics 2018: GLOBOCAN Estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018; 0:1-31
James L, David M, Sandra D, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–90.
Achim R, Fabrice B, Daniel W, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65.
Hossein B, Luis P-A, Leora H, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Ferris Robert L, George B, Jerome F, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242–50.
Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8.
E. Sa^ada-Bouzid, C. Defaucheux, A. Karabajakian et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017; 28(7): 1605–11
Stéphane C, Roberto F, Christophe M, Benjamin B, Aurélien M, Jean-Charles S, Charles F. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15:748–62.
Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–52.
Siefker-Radtke A, Curti B. Immunotherapy in metastatic urothelial carcinoma: focus on immune checkpoint inhibition. Nat Rev Urol. 2018;15(2):112–24.
Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book. 2018;38:169–78.
Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025. Eur Urol. 2017;72(3):368–76.
Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v.11 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13):1510–7.
Borcoman Edith, Nandikolla Amara, Long Georgina, Goel Sanjay, Le Tourneau Christophe. Patterns of response and progression to immunotherapy. Am. Soc. Clin. Oncol. Educ. 2018; 38: 169–78.
Fuentes-Antrás J, Provencio M, Dí az-Rubio E. Hyperprogression as a distinct outcome after immunotherapy. Cancer treatment reviews. 2018; 70: 16–21.
Jong Yeob Kim, Keum Hwa Lee, Jeonghyun Kang et al. Hyperprogressive Disease during Anti-PD-1 (PDCD1)/PD-L1 (CD274) Therapy: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019; 11(11): 1699.
G. Wells., B. Shea., D. O'Connell., J. Peterson., V. Welch., M. Losos., P. Tugwell. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [cited 2017 January 12].
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16.
Aoki M, Shoji H, Nagashima K, et al. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open. 2019;4(3):e000488.
Kim CG, Kim KH, Pyo K-H, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104–13.
Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22(4):793–802.
Kanjanapan Y, Day D, Wang L, et al. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer. 2019;125(8):1341–9.
Ji Z, Peng Z, Gong J, Zhang X, Li J, Ming Lu, Zhihao Lu, Shen L. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer. 2019;19(1):705.
Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25(3):989–99.
Tunali I, Gray JE, Qi J, Abdalah M, Jeong DK, Guvenis A, Gillies RJ, Schabath MB. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: an early report. Lung cancer (Amsterdam, Netherlands). 2019;129:75–9.
Kim Y, Kim CH, Lee S, Lee HY, Kim HS, Kim K, Sun J, Ahn JS, Ahn M, Park K. Clinical and genetic characterization of hyperprogression based on volumetry in advanced NSCLC treated with immunotherapy. J Thorac Oncol. 2019;14(9):1608–18.
Fang W, Zhou H, Shen J, Li J, Zhang A, Hong S, Zhang L. MDM2/4 amplification predicts poor response to immune checkpoint inhibitors: a pan-cancer analysis. ESMO Open. 2020;5(1):e000614.
Forschner A, Hilke F-J, Bonzheim I, et al. MDM2, MDM4 and EGFR amplifications and hyperprogression in metastatic acral and mucosal melanoma. Cancers. 2020;12(3):540.
Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19:737–46.
Takatsugu O, Hironaga S, Misato O, Yukimasa H, Hisateru Y. Hyperprogressive disease in the irradiation field after a single dose of nivolumab for gastric cancer: a case report. Case Rep Oncol. 2018;11(1):143–50.
Fields EC, McGuire WP, Lin L, Temkin SM. Radiation treatment in women with ovarian cancer: past, present, and future. Front Oncol. 2017;7:177.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
XX contributed to the study conception and design. Material preparation, data collection and analysis were performed by JL, QW, SW and XX. The first draft of the manuscript was written by JL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Wu, Q., Wu, S. et al. Investigation on potential biomarkers of hyperprogressive disease (HPD) triggered by immune checkpoint inhibitors (ICIs). Clin Transl Oncol 23, 1782–1793 (2021). https://doi.org/10.1007/s12094-021-02579-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02579-9