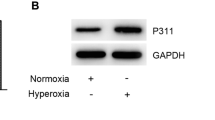
Abstract
Transdifferentiation of alveolar epithelial type II cells (AECIIs) to type I cells (AECIs) is critical for reestablishment and maintenance of an intact alveolar epithelium. However, this process is frequently destroyed by hyperoxia treatment, which is commonly used in respiratory distress syndrome therapy in preterm infants. Wnt5a is considered to participate in this physiopathologic process, but the clear mechanisms still need to be further investigated. In this study, preterm rats and primary rat AECIIs were exposed to hyperoxia. Hematoxylin and eosin staining was used to examine the histological changes of the lungs. Real-time PCR and western blotting were used to examine Wnt5a expression and biomarkers of AECII and AECI expression. Immunohistochemistry and immunofluorescence were also used to determine the expression and location of selected biomarkers. Furthermore, AECIIs transfected with Wnt5a gene and exogenous Wnt5a were used to examine whether Wnt5a contributes to the transdifferentiation of AECIIs to AECIs. Results showed that hyperoxia inhibited the transdifferentiation of AECIIs to AECIs in vitro, which is represented by biomarkers of two types of cell that remained unchanged. In addition, Wnt5a protein expression was found to be decreased after hyperoxia exposure in vitro and in vivo. Furthermore, both the overexpression of Wnt5a and exogenous Wnt5a addition blocked the inhibitory effect of hyperoxia in vitro. In conclusion, our results suggest that the transdifferentiation of AECIIs to AECIs is impaired by hyperoxia, and this process may be associated with Wnt5a downregulation. Targeting Wnt5a may have the potential for the therapy of lung injury in preterm infants induced by hyperoxia.









Similar content being viewed by others
References
Adamson IY, Bowden DH (1974) The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30:35–42
Akella A, Deshpande SB (2013) Pulmonary surfactants and their role in pathophysiology of lung disorders. Indian J Exp Biol 51:5–22
Ballard PL (1989) Hormonal regulation of pulmonary surfactant. Endocr Rev 10:165–181
Barth K, Blasche R, Kasper M (2010) T1alpha/podoplanin shows raft-associated distribution in mouse lung alveolar epithelial E10 cells. Cell Physiol Biochem 25:103–112
Beers MF, Shuman H, Liley HG, Floros J, Gonzales LW, Yue N, Ballard PL (1995) Surfactant protein B in human fetal lung: developmental and glucocorticoid regulation. Pediatr Res 38:668–675
Bhaskaran M, Xi D, Wang Y, Huang C, Narasaraju T, Shu W, Zhao C, Xiao X, More S, Breshears M, Liu L (2012) Identification of microRNAs changed in the neonatal lungs in response to hyperoxia exposure. Physiol Genomics 44:970–980
Bishop AE (2004) Pulmonary epithelial stem cells. Cell Prolif 37:89–96
Boucherat O, Franco-Montoya ML, Thibault C, Incitti R, Chailley-Heu B, Delacourt C, Bourbon JR (2007) Gene expression profiling in lung fibroblasts reveals new players in alveolarization. Physiol Genomics 32:128–141
Cao YX, Ramirez MI, Williams MC (2003) Enhanced binding of Sp1/Sp3 transcription factors mediates the hyperoxia-induced increased expression of the lung type I cell gene T1alpha. J Cell Biochem 89:887–901
Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS (2008) Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun 365:285–290
Cohen JC, Larson JE, Killeen E, Love D, Takemaru K (2008) CFTR and Wnt/beta-catenin signaling in lung development. BMC Dev Biol 8:70
Crapo JD, Barry BE, Foscue HA, Shelburne J (1980) Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122:123–143
Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER (1982) Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126:332–337
De Paepe ME, Mao Q, Chao Y, Powell JL, Rubin LP, Sharma S (2005) Hyperoxia-induced apoptosis and Fas/FasL expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 289:L647–L659
Downs CA, Montgomery DW, Merkle CJ (2011) Cell culture models using rat primary alveolar type I cells. Pulm Pharmacol Ther 24:577–586
Eber E, Zach MS (2001) Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 56:317–323
Evans MJ, Cabral LJ, Stephens RJ, Freeman G (1975) Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22:142–150
Ghosh MC, Gorantla V, Makena PS, Luellen C, Sinclair SE, Schwingshackl A, Waters CM (2013) Insulin-like growth factor-I stimulates differentiation of ATII cells to ATI-like cells through activation of Wnt5a. Am J Physiol Lung Cell Mol Physiol 305:L222–L228
Gomperts BN, Strieter RM (2007) Stem cells and chronic lung disease. Annu Rev Med 58:285–298
Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L (2005) Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 288:L179–L189
Gonzalez RF, Allen L, Dobbs LG (2009) Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 297:L1045–L1055
Guttentag SH, Beers MF, Bieler BM, Ballard PL (1998) Surfactant protein B processing in human fetal lung. Am J Physiol 275:L559–L566
Hou A, Fu J, Yang H, Zhu Y, Pan Y, Xu S, Xue X (2015) Hyperoxia stimulates the transdifferentiation of type II alveolar epithelial cells in newborn rats. Am J Physiol Lung Cell Mol Physiol 308:L861–L872
Hsia CC, Hyde DM, Ochs M, Weibel ER, Structure AEJTFoQAoL (2010) An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med 181:394–418
Ikehata M, Yumoto R, Nakamura K, Nagai J, Takano M (2008) Comparison of albumin uptake in rat alveolar type II and type I-like epithelial cells in primary culture. Pharm Res 25:913–922
Jobe AH, Kallapur SG (2010) Long term consequences of oxygen therapy in the neonatal period. Semin Fetal Neonatal Med 15:230–235
Johnston LC, Gonzales LW, Lightfoot RT, Guttentag SH, Ischiropoulos H (2010) Opposing regulation of human alveolar type II cell differentiation by nitric oxide and hyperoxia. Pediatr Res 67:521–525
Katoh M (2008) WNT signaling in stem cell biology and regenerative medicine. Curr Drug Targets 9:565–570
Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G (2005) Wnt-5a has tumor suppressor activity in thyroid carcinoma. Oncogene 24:2144–2154
Leris AC, Roberts TR, Jiang WG, Newbold RF, Mokbel K (2005) WNT5A expression in human breast cancer. Anticancer Res 25:731–734
Li C, Xiao J, Hormi K, Borok Z, Minoo P (2002) Wnt5a participates in distal lung morphogenesis. Dev Biol 248:68–81
Li Z, Fang F, Xu F (2013) Effects of different states of oxidative stress on fetal rat alveolar type II epithelial cells in vitro and ROS-induced changes in Wnt signaling pathway expression. Mol Med Rep 7:1528–1532
Liang H, Chen Q, Coles AH, Anderson SJ, Pihan G, Bradley A, Gerstein R, Jurecic R, Jones SN (2003) Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4:349–360
Liu XX, Yu XR, Jia XH, Wang KX, Yu ZY, Lv CJ (2013) Effect of hyperoxia on the viability and proliferation of the primary type II alveolar epithelial cells. Cell Biochem Biophys 67:1539–1546
Liu A, Chen S, Cai S, Dong L, Liu L, Yang Y, Guo F, Lu X, He H, Chen Q, Hu S, Qiu H (2014) Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS One 9:e90229
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Lu H, Chang L, Li W, Jiang N, Peng Q, Cai C, Liu J (2007) Effects of hyperoxia on the dynamic expression of Aquaporin5 in premature rats lung development. J Huazhong Univ Sci Technol Med Sci 27:318–320
Lu HY, Shao GB, Li WB, Wang H (2011) Effects of hyperoxia on transdifferentiation of primary cultured type II alveolar epithelial cells from premature rats. In Vitro Cell Dev Biol Anim 47:64–72
Maniscalco WM, Watkins RH, O'Reilly MA, Shea CP (2002) Increased epithelial cell proliferation in very premature baboons with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 283:L991–L1001
Mason RJ, Kalina M, Nielsen LD, Malkinson AM, Shannon JM (2000) Surfactant protein C expression in urethane-induced murine pulmonary tumors. Am J Pathol 156:175–182
Nielsen S, King LS, Christensen BM, Agre P (1997) Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am J Physiol 273:C1549–C1561
Northway WH Jr, Rosan RC, Porter DY (1967) Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276:357–368
Pongracz JE, Stockley RA (2006) Wnt signalling in lung development and diseases. Respir Res 7:15
Pukrop T, Klemm F, Hagemann T, Gradl D, Schulz M, Siemes S, Trumper L, Binder C (2006) Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A 103:5454–5459
Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC (2003) T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol 256:61–72
Roan E, Wilhelm K, Bada A, Makena PS, Gorantla VK, Sinclair SE, Waters CM (2012) Hyperoxia alters the mechanical properties of alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 302:L1235–L1241
Roper JM, Mazzatti DJ, Watkins RH, Maniscalco WM, Keng PC, O'Reilly MA (2004) In vivo exposure to hyperoxia induces DNA damage in a population of alveolar type II epithelial cells. Am J Physiol Lung Cell Mol Physiol 286:L1045–L1054
Slusarski DC, Corces VG, Moon RT (1997) Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390:410–413
Smith LJ, McKay KO, van Asperen PP, Selvadurai H, Fitzgerald DA (2010) Normal development of the lung and premature birth. Paediatr Respir Rev 11:135–142
Sola A, Rogido MR, Deulofeut R (2007) Oxygen as a neonatal health hazard: call for détente in clinical practice. Acta Paediatr 96:801–812
Thebaud B, Abman SH (2007) Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 175:978–985
Wert SE, Glasser SW, Korfhagen TR, Whitsett JA (1993) Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol 156:426–443
Xu W, Yang N, Pan L, Fu J, Xue X (2012) The expression of HoxB5 and its role in neonatal rats with chronic lung disease. Fetal Pediatr Pathol 31:11–20
Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA (2006) Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291:L1101–L1111
Yee M, Buczynski BW, O'Reilly MA (2014) Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells. Am J Respir Cell Mol Biol 50:757–766
Yu XM, Wang L, Li JF, Liu J, Li J, Wang W, Wang J, Wang C (2013) Wnt5a inhibits hypoxia-induced pulmonary arterial smooth muscle cell proliferation by downregulation of beta-catenin. Am J Physiol Lung Cell Mol Physiol 304:L103–L111
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 81270726).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
The experiment was designed by Wei Xu. The manuscript was written by Wei Xu and Bo Xu. The experiment was performed by Bo Xu, Ying Zhao, Ni Yang, Chunfeng Liu, Guangfu Wen, and Binglun Zhang. The data were analyzed by Wei Xu and Bo Xu.
Rights and permissions
About this article
Cite this article
Xu, W., Xu, B., Zhao, Y. et al. Wnt5a reverses the inhibitory effect of hyperoxia on transdifferentiation of alveolar epithelial type II cells to type I cells. J Physiol Biochem 71, 823–838 (2015). https://doi.org/10.1007/s13105-015-0446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0446-4